
What is Micropropagation?
- As the alternative means of plant vegetative propagation, tissue culture techniques are becoming increasingly popular. Plant tissue culture consists of asexual methods of propagation and its primary objective is crop improvement.
- The success of many invitro selection and genetic manipulation techniques in higher plants relies on the success of invitro plant regeneration.
- Clonal propagation invitro is called micropropagation.
- In micropropagation, apical meristem is cultivated. so this technique is also known as meristem culture or mericlones.
- Webber used the word ‘clone’for first time to apply for cultivated plants that were propagated vegetatively.
- It indicates that plants grown from such vegetative parts are not individuals in the ordinary sense, but are simply transplanted parts of the same individual and such plants are identical.
- Thus, the multiplication of genetically identical individuals by asexual reproduction is termed as clonal propagation whereas plant population derived from a single individual by asexual reproduction is termed as clone.
- The current techniques of single cell and protoplast culture allows many thousands of plants to be ultimately derived from a single cell in a comparatively short span of time.
- Thus, the products of this rapid vegetative propagation should by definition be considered a single clone.
- The aseptic methods of clonal propagation provide major advantage over the conventional methods that in a relatively short span of time and space, a large number of plants can be produced starting from a single individual.
- Axillary bud proliferation approach is estimated to yield an average 10-fold increase in shoot number per monthly culture passage.
- In a period of 6 months, it is likely to obtain as many as 1,000,000 propagules or plants starting from a single explant.
- Suitable explants from vascular plants including angiosperms, gymnosperms and pteridophytes can be cultured in vitro and induced to form adventitious buds, shoots, embryoids or whole plants.
Stages of micropropogation
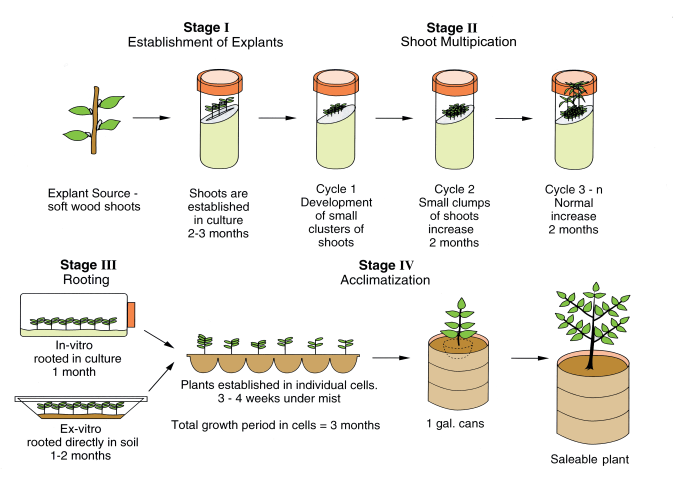
Murashige (1974) outlined three major stages involved in Micropropagation.
Stage 0:
- This involves, selection of suitable healthy stock plant from which explants can be obtained.
Stage I:
- It involves selection of suitable explants, their sterilization and transfer to nutrient media for establishment, i.e. initiation of a sterile culture of the explant.
- Selection of explant: At first apical meristem is excised from healthy stock plant and then sectioned by sterile blade into 5-10 mm sized explants.
- Sterilization of explant: After explant of appropriate size is obtained, it is placed in watch glass for sterilization. 70% alcohol, sodium hypochlorite and calcium hypochlorite can be used as sterilizing agents. Sterilization should be done for about appropriate time for 5-10 minutes. If the explant is exposed to sterilizing agent for longer time, cells may be killed. After sterilization, excess sterilizing agent is removed by washing the explants for several time with distilled water.
Stage II:
- This involves inoculation of explants into suitable culture media and proliferation or multiplication of shoots from the explant on medium.
- Musashige and skoog (MS) medium can be used as cultivation media.
- The inoculums are incubated for about 2 months at optimun temperature (20-25° C). During this period, shoot will be obtained.
- The shoot can be transferred into multiple shoot inducing media and incubated for sufficient time to obtain multiple shoots.
Stage III:
- This involves separation of individual shoot and transfer of shoots to a separate rooting medium followed later by planting into soil
- At the end of this stage, small plantlet with root and shoot are obtained in test tube.
Stage IV
- This involves acclimatization of plantlet before cultivation in soil outside.
- The lab grown plantlet may fail to survive in soil due to desication, unfavourable condition and due to unadjustability from dependent to independent nutrition. Therefore, acclimatization is needed before cultivation in soil.
- Acclimatization can be done –
- by exposing the test tube culture to unfavourable environment before first two week of planting outside.
- functional leaf
- by inducing functional root
Further, not all crop species need to be propagated in vitro by means of all three stages. These stages have been designed simply to describe micropropagation processes and to facilitate comparison between two or more systems.
Types of Micropropagation:
Micropropagation and plant regeneration can be classified into the following groups:
Group I: Meristem culture
- Improved release of axillary bud proliferation: i.e. by multiplication through growth and proliferation of existing meristems.
- It can be through apical shoots excised from the parent plant (meristem and shoot tip culture) and by multiplication of existing meristems within axillary shoots, which proliferate on explants after removal from the parent plant (single node and axillary bud culture).
Group II: Callus culture
- The formation of individual organs such as shoots and roots either directly on the explant where a preformed meristem is absent or de novo origin from callus and cell culture generated from the explant is termed as organogenesis.
Group III: Embryo culture
- The formation of a bipolar structure including both shoot and root meristems either of the adventitive origin i.e. directly from the explant or de novo origin from callus and cell culture induced from the explant is termed as somatic embryogenesis.
- In this culture, explant is cultivated in suitable embryo inducing medium and then embryo is cultivated into rooting and shooting medium to obtain whole plant.
Advantages of micropropagation:
- In vitro micropropagation techniques are now often preferred to conventional practices of asexual propagation because of the following advantages not only from commercial point of view but also with regard to crop improvement program:
- A small amount of plant tissue is needed as the initial explant for regeneration of millions of clonal plants in one year. In comparison it would take years to propagate an equal number of plants by conventional methods.
- Many plant species are highly resistant to conventional bulk propagation practices. Micropropagation techniques provide a possible alternative for these species. Micropropagation helps in bulking up rapidly new cultivars of important trees that would otherwise take many years to bulk up by conventional methods.
- The invitro techniques provide a method for speedy international exchange of plant materials. If handled properly, the sterile status of the culture eliminates the danger of disease introduction. Thus, the period of quarantine is reduced or unnecessary.
- The invitro stocks can be quickly proliferated at any time of the year. Also, it provides year round nursery for ornamental, fruit and tree species.
- Production of disease free plants:
- Meristem tip culture is generally employed in cases where the aim is to produce disease-free plants.
- It has been demonstrated that the shoot apices of virus-infected plants are frequently devoid of viral particles or contain very low viral concentration.
- Though chemotherapeutic and physical agents have been used for production of virus-free plants but with limited success.
- Invitro culture has become the only effective technique to obtain virus-free plants in potato, Dianthus, chrysanthemum, gladiolus, Pelargonium, sweet potato (Ipomea batatus), yam (Dioscorea rotundata), cassava (Manihot esculenta), etc. from stocks systemically infected not only with virus but with various other pathogens.
- Seed production:
- A major limiting factor for seed production in some of the crops is requirement of the high degree of genetic conservation.
- In such cases micropropagation (axillary bud proliferation method) can be used. For example, production of F1 hybrid seed lines in crops like cauliflower where individual parent clones can be bulked for the production of more uniform seed, the production of male sterile lines in crops like onion to provide an alternative to difficult backcrossing methods, and the production of asparagus for producing high quality supermale and female homozygous lines from which desirable all male hybrids can be produced.
- Germplasm conservation:
- Plant breeding programmes rely heavily on the germplasm.
- Preservation of germplasm is a means to assure the availability of genetic materials as the need arises.
- Most seeds and vegetative organs have a limited storage life, research on germplasm preservation has concentrated on the development of procedures to extend usable life spans.
- Since meristem cells are highly cytoplasmic and non-vacuolated, a high percentage of cells can be expected to survive cryopreservation procedures.
- Meristems are genetically stable and can be regenerated into pathogen-free plants. Meristems have been identified as excellent material for germplasm preservation of crop species with seed borne viruses also.
- Production of Artificial seeds:
- The concept of artificial or synthetic seeds (i.e. encapsulated embryos) produced by somatic embryogenesis has become popular.
- The aim of somatic embryo (SE) encapsulation is to produce an analog to true seeds, which is based on the similarity of SE with zygotic embryo with respect to gross morphology, physiology and biochemistry.
- Two types of synthetic seeds have been developed, namely, hydrated and desiccated.
Limitations of micropropagation:
- Expensive requirements and sophisticated facilities with trained manpower are needed.
- Though precautions of high order are normally taken during culture, but there are chances of contamination by various pathogens. Contamination could cause very high losses in a short time.
- The problem of genetic variability may be pronounced in some forms of culture. Shoot tip culture tends to remain stable while those systems that utilize adventitious shoots or multiplication of callus, genetic variability is pronounced.
- Source of variability may be due to chimeral breakdown, aberrant cell division in callus or cell suspension, epigenetic effects and pre-existing genetic variability.
- It has been experienced that during repeated cycles of invitro shoot multiplication, cultures show water-soaked, almost translucent leaves.
- Such shoots exhibit a decline in the rate of growth multiplication, become necrotic and may eventually die. This phenomenon is referred as vitrification.
- This is also known as hyperhydration. |
- This is due to morphological, physiological and metabolic dearrangements occurring during intensive shoot multiplication in culture.
- Some preventive measures to reduce hyperhydration includes:
- increasing concentration of agar
- overlaying medium with paraffin
- using desiccant such as CuSO4
- bottom cooling of the culture vial to improve aeration
- lowering cytokinin level or replacing one kind of cytokinin by another
- manipulating NH + or salt concentrations in the culture medium.
