
What are the different types of plant tissue culture?
Following are types of plant tissue culture:
- Cell or suspension culture
- Explant culture
- Callus culture
- Protoplast culture
- Embryo culture
- Anther and pollen culture
- Ovule culture
- Ovary culture
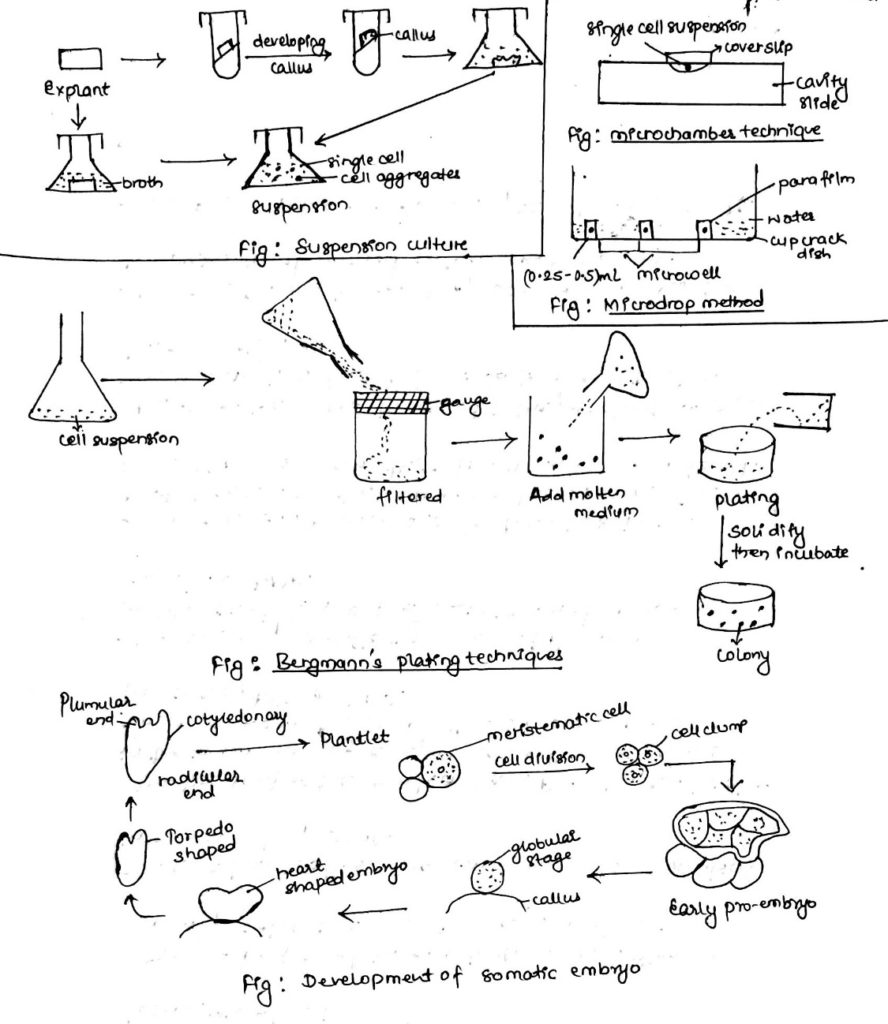
1. Cell or suspension culture:
- Cell or suspension culture can be obtained either directly from explant or from callus.
- Pieces of undifferentiated calli are transferred to liquid medium which is continuously agitated to obtain a suspension culture.
- Tissue and cells cultured in a liquid medium produces a suspension of single cells and cell clumps of few to many cells which is called suspension culture.
- Single cells can also be obtained from plant organs (explants) particularly from leaf either by mechanical or enzymatic (pectinase solutions) means.
- Then single cells can be cultured by different techniques.
- Single cell culture methods:
- Filter paper raft nurse tissue technique:
- Single cells are placed on filter paper (8X8mm) usually which themselves are placed on top of established callus cultures.
- Single cells will be able to derive nutrient from callus exudates diffusing through the filter papers.
- Microchamber technique:
- A microchamber can be created either by using a microscopic slide and coverslip or a cavity slide.
- Single cells are suspended in a condition medium and the drop is placed onto a coverslip which is then inverted into the slide cavity.
- This method allows microscopic observation that can be sub-cultured on petri dish easily.
- Microdrop method:
- An especially designed dish (cup rack dish) having a smaller outer chamber to be filled with distilled water to avoid dessications of cells and a larger chamber having several microncells is used.
- Microdrops of (0.25-0.5) ml are distributed in the micron-cells and the dish is sealed with parafilm.
- Cell density in the medium is adjusted to give on an average one cell per droplet.
- Bergmann’s plating technique:
- In this technique, free cells are suspended in a liquid medium and if cell aggregates are present, these are filtered.
- Culture medium with (0.8-1)% is cooled and maintained at 35oC in a water bath.
- Then cells are mixed with the medium and poured in a petri dish.
- Then, it is allowed to solidify and sealed with parafilm and examined with inverted microscope to mark single cells.
- When macroscopic colonies develop, they are isolated and cultured separately.
Applications of single cell:
- Induction of somatic embryos and shoots.
- Invitro mutagenesis and selection of mutants.
- Genetic transformation studies.
- Production of secondary metabolites.
2. Explant culture:
- Any excised part of plant which has capacity or capability to develop into a whole plant is called explant.
- Explant should contain parenchyma tissues.
- Parenchyma tissues are versatile and capable of division and growth so far explant culture, the explant we have selected must contain parenchyma tissue.
- Explant should be selected from young and healthy part of plant.
- It can be from stems, rhizomes, tubers, roots, cotyledons, and hyper cotyledons.
- Development of the tissue occur through cell division, cell elongation, and cell differentiation.
3. Callus culture:
- Callus is an amorphous mass of loosely arranged, thin-walled parenchyma cells developing from proliferating cells of the parent tissue.
- It has biological potential to develop normal root, shoot and embryos and ultimately forming a plant.
- Callus culture should be subcultured in every 28 days to prevent nutrient depletion and production of toxic metabolites.
- Factors:
- Environmental factors
- Nutrient composition (Concentration of auxins and cytokinins)
- Explant source
4. Somatic embryogenesis:
- Development of embryo from somatic or vegetative cells is somatic embryogenesis.
- Zygotic embryo:
- Embryo formed from zygote.
- Non-zygotic embryo:
- Embryo derived from other parts than zygote.
- Somatic embryo:
- Embryo derived from somatic or vegetative parts.
- Parthenogenic embryo:
- Embryo derived from unfertilized egg is known as parthenogenic embryo.
- Androgenic embryo:
- Embryo derived from pollen.
- In 1959, from the callus culture and suspension culture of carrot (Daucus carota), somatic embryogenesis was done for first time.
- Major nutrient factor for somatic embryogenesis is auxin (high concentration is taken) and smaller amount of cytokinin may also be used.
Characteristics of somatic embryogenesis:
- Are bipolar in nature i.e., it has radicle and plumule end. (Radicle: from where roots are regenerated and plumule end: shoots are regenerated)
- Somatic embryo can be developed from two different cells.
- Dedifferentiation:
- Dedifferentiation can be defined as reversion process in which mature meristematic step of cell lead to the formation of callus.
- Mature cell ——–> callus
- Redifferentiation:
- Component of callus have the ability to grow differentiated cells or plantlet.
- This process is known as redifferentiation.
- Organogenesis:
- Organogenesis can be defined as development of organs (shoot, root, leaves) that are induced in plant tissue culture.
- It can also be known as regeneration.
5. Protoplast culture:
- Protoplast is a cell without cell wall.
- It is important for culture of hybrid plants.
- Hybrid: The fusion of two cells of two different plants.
- Cybrid: The fusion of the cell of nucleated plant and unnucleated cell.
- Protoplast is a cell from which cell wall is removed.
- This can be done either mechanically or enzymatically or by combination of both.
- CaCl2 is used to stabilize plasma membrane.
Stages of protoplast culture:
I. Isolation of protoplast:
- Protoplast can be isolated from variety of tissue including leaves, root, invitro shoot callus, cell suspension and pollen but most commonly used substances are leaves.
- The process of isolation of protoplast from leaves are as follows:
Steps of protoplast isolation:
- Sterilization of leaf/leaves:
- Rinse/ wash with clean water to remove dust particles/ soil particles.
- Dip in 70% ethyl alcohol and 2% solution of sodium hypochlorite for few minutes.
- Rinse/wash with sterile distilled water few times (3-4 times) to remove sodium hypochlorite.
- Removal of epidermis:
- Remove lower epidermis gently and cut leaves into small pieces (for early removal of protoplast).
- Enzymatic treatment:
- This can be performed in two different ways:
- Direct (One step) method:
- 0.5% macerozyme or pectinase + 2% cellulase in 13% mannitol or sorbitol at pH 5.4 at (25-30)oC for few hours.
- Teased gently and try to isolate protoplast.
- Sequential (Two step) method:
- 0.5% macerozyme or pectinase enzyme is taken.
- Along with it, 0.3% potassium dextran (antioxidant) is added in 10% mannitol or sorbitol solution.
- Place in water bath maintained at 25oC and by shaking for 15 minutes.
- Add fresh enzyme and incubate for an hour.
- After removing previous enzyme solution, wash with 13% mannitol or sorbitol.
- Add enzyme B (2% cellulase in 13% sorbitol or mannitol)
- Incubate for few hours.
- Purification:
- Filter the solution obtained from enzymatic treatment in 45 μ Nylon mesh and take the filtrate.
- For further purification, centrifuge filtrate at 100g for 1 minute.
- Remove supernatant.
- Wash pellet 3 times (13% mannitol or sorbitol, centrifuge 100g for 1 minute).
- Resuspend pellet in 20% sucrose solution.
- Centrifuge at 200g for 1 minute.
- Pure protoplast form supernatant.
- Then separate protoplast by using pipette.
II. Culture:
- Make 105 cell (protoplast)/ml solution with mannitol or sorbitol.
- Culture by Bergmann’s culture technique or microchamber technique or microdrop method.
- First callus is obtained which is sub-cultured as plantlet.
Applications of protoplast culture:
- Biochemical and metabolic studies
- Production of hybrid plants (nucleus of two different plants fused)
- Production of cybrid (fusion of nucleated and enucleated cells).
- Genetic manipulation
- To test during sensitivity
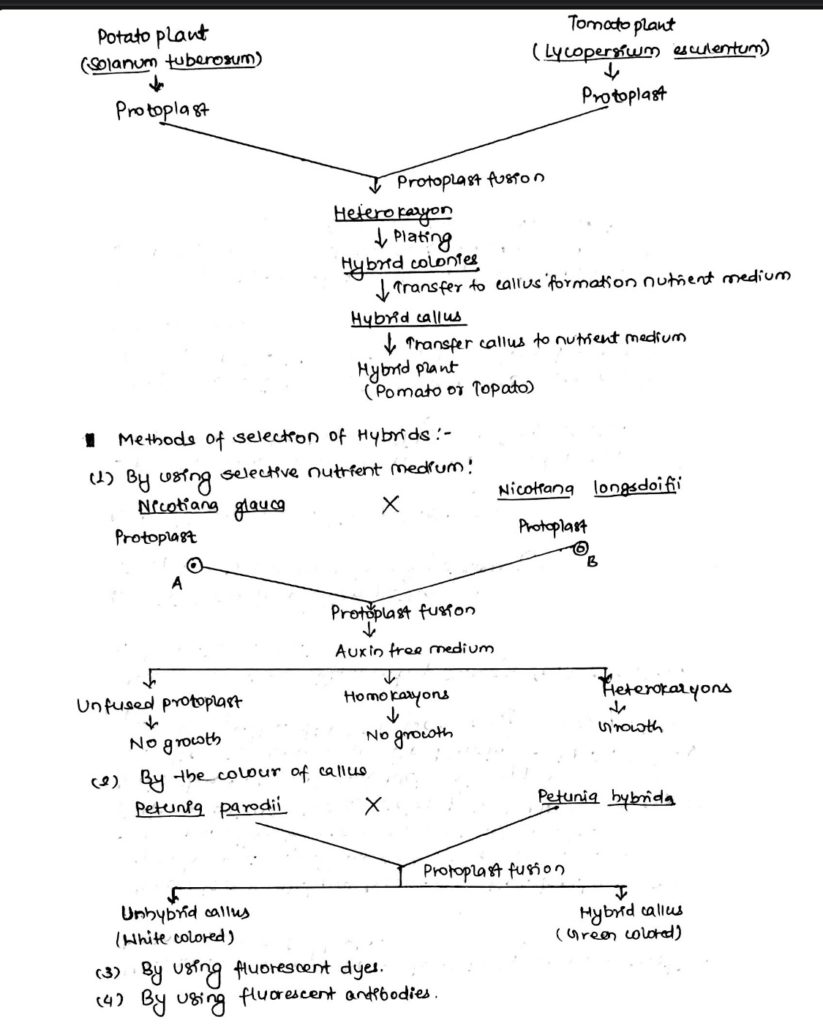
Somatic hybridization:
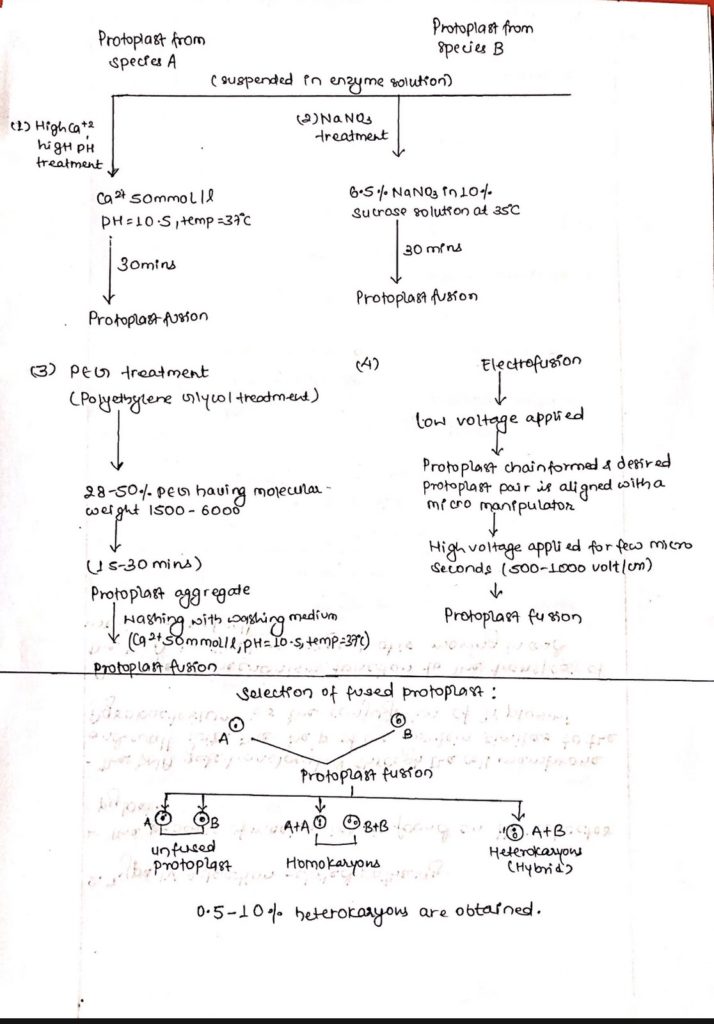
- Production of hybrid plants through the fusion of protoplast of two different plant species or varieties is called somatic hybridization and the hybrid plant obtained is known as somatic hybrid stages involved in somatic hybridization are:
- Isolation of protoplast of two different plant species or varieties.
- Mixing of protoplast by Fusogen treatment (NaNO3, PEG TR, Ca++ treatment, electrofusion).
- Wall regeneration by heterokaryotic cells (having two nucleus from two different species or two different varieties).
- Fusion of nuclei of heterokaryon to produce hybrid cells.
- Plating and production of colonies of hybrid cells.
- Selection of hybrid subculture and plantlet regeneration.
Protoplast fusion (different methods used in protoplast fusion (Induced fusion).
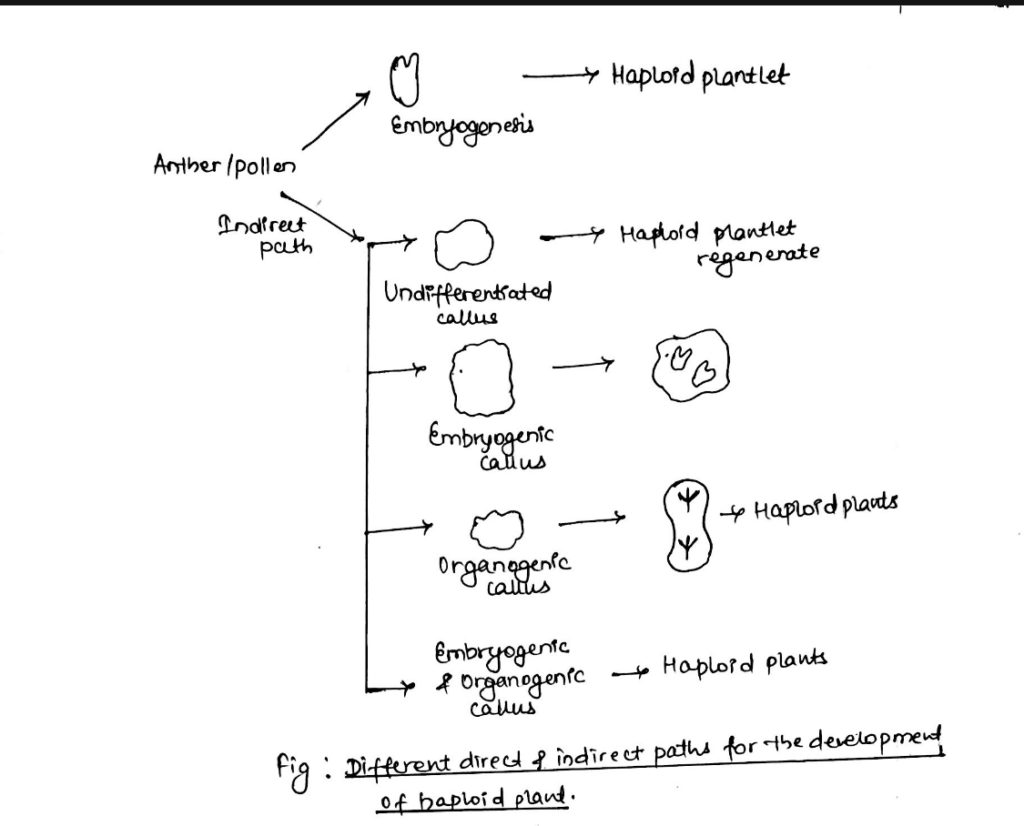
6. Anther and Pollen culture:
- The objectives of anther and pollen culture is:
- For the production of haploid plants (having ‘n’ number of chromosomes) to produce same type of plants to stable genetic variability.
- Haploids can be defined as sporophyte with gametophytic chromosome number which have a single complete set of chromosomes that may be useful for the improvement of many crop plants.
- Anther and pollen or microspore culture are popular methods for the production of haploid plants.
- Parameters for successful anther and pollen cultures:
- Conditions of growth of donor plant
- Genotype of donor plant
- Pre-treatment
- The developmental stage of anther or pollen
- The culture medium and the environmental conditions during growth.
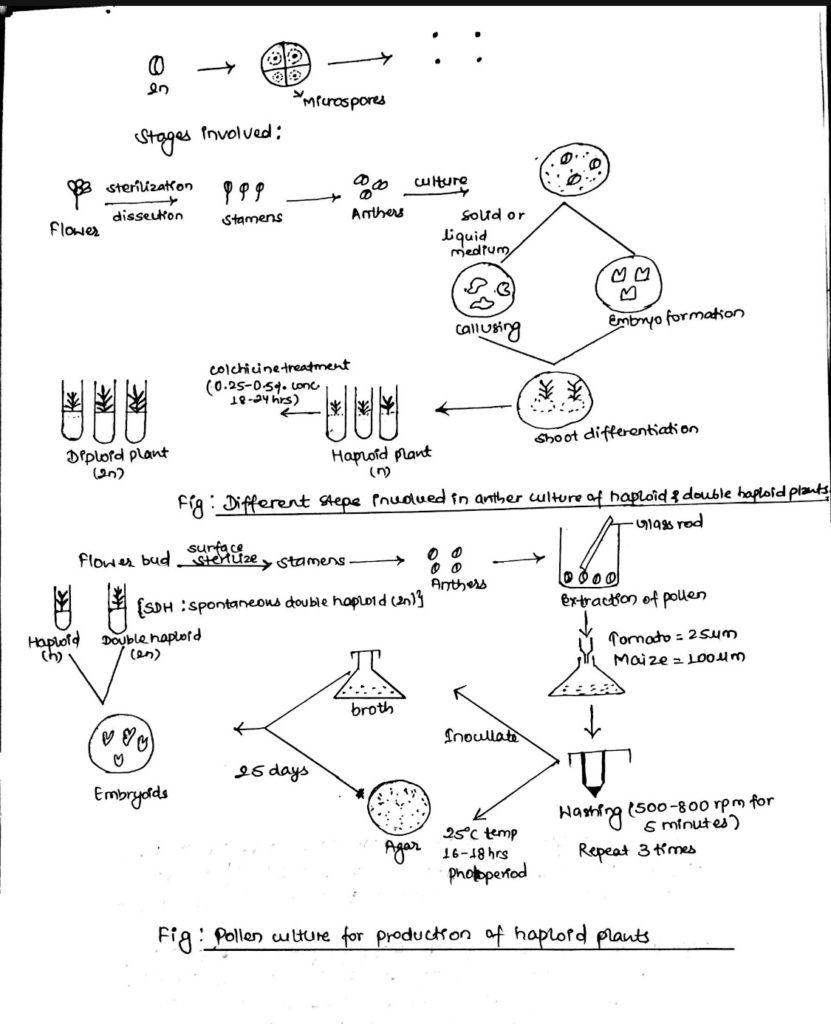
i. Anther culture:
- Anther is a male reproductive organ having diploid in chromosome number.
- As a result of microsporogenesis, tetrads of microspores are formed from a single mother spore, they are known as pollen grains after released from tetrads.
- For anther culture, flower buds are brought to laminate air flow chamber and sterilized using appropriate chemical treatment.
- While removing anthers from flower buds, care should be taken to avoid injury because injury may lead to the development of callus which gives the mixture of haploids and diploids.
ii. Pollen culture:
- Pollen culture is preferred over anther culture even though the degree of success is low in this case
- About 50 anthers is placed in 20ml medium squeezed with a glass rod or syringe piston to allow the microspore to squeeze out.
- This solution (suspension of haploids and diploids) is filtrated through nylon sieve which allows only the microspore to pass through.
- The filtrate is centrifuged 3 time for 5 mins each at (500-800) rpm.
- The microspores are inoculated on solid or liquid medium maintained at 25oC for (16-18) hrs photoperiod.
- The microspores may develop directly into embryoids within 15 days or follow one of the several indirect path to reproduce haploid plantlets.
- In anther culture as well as pollen culture, spontaneous double haploid may also be obtained which does not require colchicine treatment.
- For culture, commonly used medium: MS, White’s medium
- Sucrose concentration: 2-3%
- For dhaturo and tobacco: (2-4)% sucrose
- Cereals: N6, Potato-2 media
- Wheat: sucrose concentration, 6%
7. Embryo culture:
- Embryo culture has been done for the production of haploid plants.
- It is used for the recovery of plants from distinct crosses and is useful where embryo fails to develop due to degeneration of embryonic tissues.
- This is extensively used in hybrid barley (Hordeum vulgare X H. bulbosum).
Procedure of embryo culture:
- Pluck or take developed fruit
- Wash it with clean water.
- Surface sterilize with 0.01% tween 20 for 15 minutes.
- Wash with distilled water few times.
- Sterilize with 0.01% mercuric chloride (HgCl2) for 10-15 minutes.
- Wash with sterile distilled water few times.
- Break seed aseptically and isolate the embryo.
- Inoculate embryo on callus proliferating media.
- After four weeks of inoculation callus appears.
- And after 8 weeks of inoculation, subculture on shoot regenerating medium.
- After 4 weeks, green callus and embryoids starts appearing.
- After 12 weeks shoot appears.
- In some case like Brassica species, embryo can be cultured on very early stage.
- Early stage embryos are heterotrophic and cannot produce required nutrients from the simple tissue culture media. So, they can be cultured in a specialized way.
- Embryo nurse endosperm technique is most commonly used method to culture embryo on very early stage.
8. Ovary culture:
- The objectives of ovary culture is;
- To overcome pre-fertilization barrier.
- To overcome post-fertilization barrier (embryo rescue).
- Culture of unfertilized or fertilized ovaries to obtain plant is called ovary culture.
- Ovary culture is also known as gymnogenesis.
- About (0.2-6)% of the cultured ovaries shown gymnogenesis and 1 or 2 rarely upto 8 plantlets originate from each ovary.
- Ovary culture is often used either for invitro pollination and fertilization or for post fertilization barrier (embryo rescue).
- Interspecific hybrid using ovary culture have been successfully obtained in several genera of Brassica (Brassica campestris X B. oleraceae).
- Technique for growing excised ovaries on culture medium was developed in many plant materials including tobacco, tomato, beans etc.
- For interspecific or intergeneric crosses, ovaries are excised at the zygote stage or two-celled pro-embryo space and normal development is completed invitro.
- By addition of different chemicals like IAA, fruit juice, coconut milk to the medium, the development is promoted.
- Limitations of ovary culture:
- Successful only in less than two dozen species.
- Success rate is very less (0.2-6)%
Applications of Haploids in plant breeding:
- Homozygous lines for the production of homozygous plant.
- Hybrid sorting in haploid breeding.
– Selection of recombinant superior gametes. - Mutation research
- Gametoclonal variation: variation in gametic chromosomal number
- Cytogenic research: Basic chromosome number
- Evolutionary studies
- Genetic studies
Applications of plant tissue culture:
- Production of secondary metabolites. E.g. alkaloids, steroids, phenolics, variety of flavors and perfumes.
- Rapid clonal multiplication or clonal propagation (which also known as micropropagation).
- Production of virus free plant (By thermotherapy, chryotherapy, chemotherapy, and meristem culture).
- Rapid development of homozygous lines by producing haploids (anther culture, ovary culture, etc.)
- Production or recovery of hybrid plants which are difficult to grow in normal conditions. (Embryo rescue, in-vitro pollination).
- Germplasm conservation. (By cryopreservation, and slow growth cultures).
- Genetic modification of plants (somaclonal variation, somatic hybridization and cybridization, gene transfer).
- Creation of genetic maps or genome maps and use of molecular markers.
- Multiplication of the plants of desired size.
- Independent of season so carried out through-out the year.
- Production of synthetic or artificial seeds which are easier to handle, transport and store.
