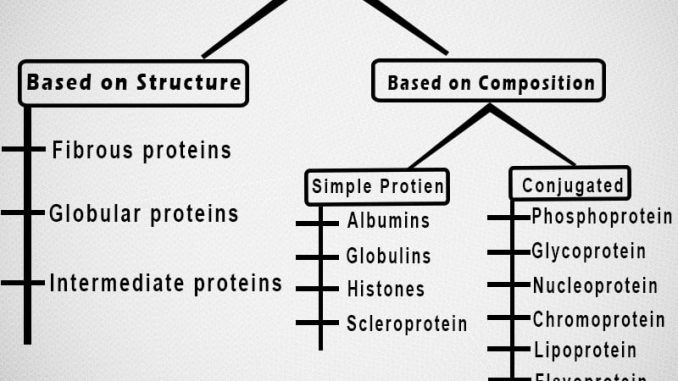
I. Classification of protein on the basis of Structure and composition:
• This Classification of protein is based on shape or structure and composition. They are classified into three types; fibrous, globular and derived protein.
1. Fibrous protein:
- They are elongated or fiber like protein.
- Axial ratio (length: breadth ratio) is more than 10
- They are static in nature with simple structure.
- They have less biological functions
- They are mostly present in animals
- Examples;
- Fibrous proteins are further classified as- simple and conjugated
- i. Simple fibrous protein:
- Examples; Scleroprotein (Keratine, elastin, collagen, fibroin etc)
- Scleroprotein or Albuminoids: they make animal skeleton and they are water insoluble.
- ii. Conjugated fibrous proteins:
- Examples; pigments present in chicken feather.
2. Globular protein:
- They are spherical or globular in shape.
- Axial ratio is always less than 10
- They are dynamic in nature (can flow or move) with higher degree of complexity in structure.
- They have variety of biological functions
- Examples; enzymes, hormones etc
- Globular protein is further classified on the basis of composition or solubility.
i. Simple or homo globular protein:
- They are composed of amino acids only.
- Some examples are;
a. Protamine:
- They are positively charged (basic) proteins mostly present in animals and fishes (sperm)
- Protamines binds with DNA in embryonic stage and later replaced by histone
- It is soluble in water and ammonium hydroxide solution
- It is not coagulated by heat
- It precipitate out in aqueous solution of alcohol
- Protamine are rich in arginine and lysine whereas devoid of sulfur containing and aromatic amino acids.
b. histone:
- They are basic protein but weak base in comparison to protamine.
- Histone is low molecular weight protein and are water soluble.
- It is not coagulated by heat.
- Histone is present in nucleic acids as nucleohistone binding with DNA.
c. Albumin:
- It is the most abundant protein in nature
- It is most commonly found in seeds in plants and in blood and muscles in animals.
- Molecular weight of albumin is 65000 KD
- It is water soluble and can be coagulated by heat
- Plant albumins; Leucosine, Legumelins etc
- Animal albumins; serum albumin, myosin, lactalbumin, ova-albumin etc
d. Globulin:
- Pseudoglobulin (water soluble) and Euglobulin (water insoluble)
e. Glutelins:
- Water insoluble. Eg. Glttenin (wheat), glutelin (corn), oryzenin (rice)
f. Prolamine:
- They are storage protein found in seeds.
- They are water insoluble. But soluble in dilute acid or detergents and 60-80% alcohol.
- They are coagulated by heat
- Prolamine is rich in proline and glutamine
- Examples; Gliadin (wheat), zein (corn), Hordein (barley), Avenin (oats)
ii. Complex or conjugate or hetero globular protein:
- These proteins in which protein are always linked by non-protein moiety to become functional. So, they are composed of both protein and non- protein components. The non-protein component is known as prosthetic group.
- On the basis of prosthetic group, they are classified as follows;
a. Metalloprotein:
- They have metal prosthetic group.
- Some metals such as Hg, Ag, CU, Zn etc, strongly binds with proteins such as collagen, albumin, casein by –SH group of side chain of amino acids.
- Eg. Ceruloplasmin; contains copper as prosthetic group
- Some other metals such as Calcium weakly binds with protein. Eg. Calsequestrin, calmodulin
- Some metals such as Na, K etc do not binds with protein but associate with nucleic acids protein.
b. Chromoprotein:
- They have colored prosthetic group.
- Some examples are;
- Haemoprotein: Haemoglobin, myoglobin, chlorophyll, cytochrome, peroxidase, haemocyanin
- Flavoprotein: Riboflavin (Vit B2) give yellow/orange color to FAD requiring enzymes
c. Glycoprotein/Mucoprotein:
- They have carbohydrate as prosthetic group
- Eg. Antibody, complement proteins, Heparin, Hyaluronic acid
d. Phosphoprotein:
- They have phosphate group as prosthetic group.
- Eg. Caesein (milk protein binds with calcium ion to form calcium salt of caseinate)
- Ovovitellin; present in egg yolk
- Calcineurin
e. Lipoprotein:
- They have lipid as prosthetic group.
- Eg. Lipovitelline, chylomicrons
3. Derived protein:
- These protein are the derivatives of either simple or complex protein resulting from the action of heat, enzymes and chemicals.
- Some artificially produced protein are included in this group.
- They are classified as primary derived protein and secondary derived protein.
i. Primary derived protein:
- The derived protein in which the size of protein molecules are not altered materially but only the arrangement is changed.
- Some examples are;
a. Proteans:
- Obtained as a first product after the action of acid or enzymes or water on protein.
- They are insoluble in water.
- Eg. Edestan, myosin
b. Metaprotein:
- They are produced by further action of acid or alkali on protein at 30-60°C.
- They are water insoluble but soluble in dil acid or alkali.
- Also known as Infraprotein.
- Eg. Curd
c. Coagulated protein:
- They are produced by the action of heat or alcohol on protein.
- They are insoluble in water.
- Eg. Coagulated egg
ii. Secondary derived protein:
- The derived protein in which size of original protein are altered.
- Hydrolysis has occurred due to which size of protein molecule are smaller than original one.
- Examples; a) Proteoses:
- They are produced by the action of dilute acid or digestive enzymes when the hydrolysis proceeds beyond the level of metaprotein.
- They are soluble in water
- They are not coagulated by heat. • Eg. Albumose, Globulose etc.
II. Classification of protein on the basis of biological functions:
- Catalytic protein:
- They catalyze biochemical reaction in cells. Eg. Enzymes and co-enzymes
2. Structural protein;
- They make various structural component of living beings.
- Eg. Collagen make bone, Elastin make ligamnets and keratin make hair and nails
3. Nutrient protein:
- They have nutritional value and provide nutrition when consumed.
- Eg. Casein in milk
4. Regulatory protein:
- They regulate metabolic and cellular activities in cell and tissue.
- Eg. Hormones
5. Defense protein:
- They provide defensive mechanism against pathogens.
- Eg. Antibodies, complement proteins
6. Transport protein:
- They transport nutrients and other molecules from one organ to other.
- Eg. Haemoglobin
7. Storage protein:
- They stores various molecules and ions in cells.
- Eg. Ferritin store Iron
8. Contractile or mobile protein:
- They help in movement and locomotion of various body parts.
- Eg. Actin, myosin, tubulin etc
9. Toxic protein:
- They are toxic and can damage tissues.
- Eg. Snake venom, bacterial exotoxins etc
