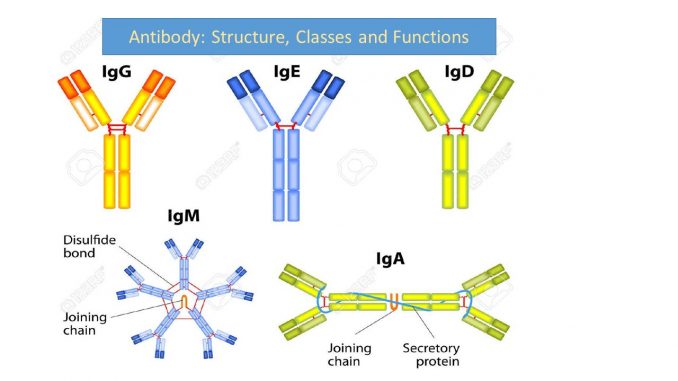
Antibody: Structure, classes and functions
Structure of antibody
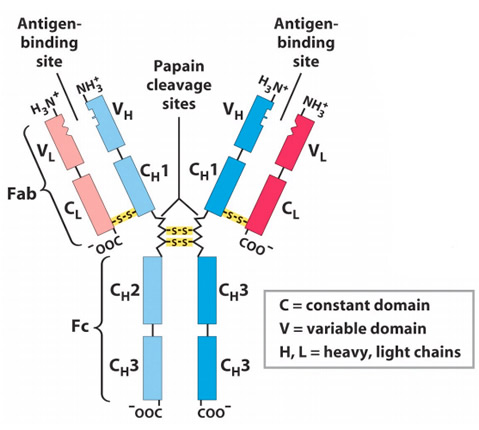
- Antibodies are the globular protein belonging to immunoglobulin (Ig) family.
- Antibody molecules have a common structure of four peptide chains. This structure consists of two identical light (L) chain polypeptide of about 22000 Da and two identical heavy (H) chain of larger polypeptide of about 55000 Da or more.
- Each light chain is bound to a heavy chain by a disulphide bond and by non-covelent interactions such as salt bride, hydrogen bonds and hydrophobic interaction to form a heterodimer (H-L). Similar non-covalent interaction and disulphide linkage link the two identical heterodimer (H-L) to each other to from basic structure of antibody ie. Dimer of dimer.
Anatomy of light (L) and heavy (H) chain:
L- chain:
- L- chain of antibody is composed of about 220 aminoacids.
- Around 100-110 aminoacids are located at N-terminal (amino-terminal) and the aminoacids sequences varies among antibodies. This region of L-chain is known as variable (V) region.
- Remaining 110 aminoacids located at C-terminal (carboxyl-terminal) of L-chain are almost constant among antibodies. This region of L-chain is known as constant (C) region. Two types of constant region sequences are found ie. Lambda (λ) and Kappa (κ). In a particular antibody either2lambda or 2 kappa chains are present but not 1 lambda and kappa.
- In human 60% light chain are kappa and 40% are lambda whereas in mice 95% of light chain are kappa and 5% are lambda.
H-chain:
- In H-chain about 110 aminoacids are located at N-terminal which shows great variation among antibody. This region is known as Variable (V) region.
- Remaining aminoacid sequences of H-chain is somewhat constant but reveals five different types of constant (C) heavy chain region ie. µ, α, δ, ε and γ.
- The length of constant region of H-chain is 330 aminoacids for α, γ and δ and 440 aminoacids for µ and ε.
Antibodies molecules are classified into five class on the basis of constant region of H-chain.
| Constant region of H-chain | antibody class | Sub-class |
| Mue (µ)
|
IgM | – |
| Gamma (γ) | IgG
|
4 sub class (IgG1, IgG2, IgG3 and IgG4) |
| Alpha (α) | IgA | 2 sub class (IgA1 and IgA2) |
| Delta (δ) | IgD | – |
| Epsilon (ε) | IgE | – |
Domain structure of antibody:
- The overall structure of immunoglobulin molecule is determined by primary, secondary, tertiary and quaternary organization of aminoacid molecules.
- The primary structure is sequence of aminoacids that comprises variable and constant region of heavy and light chain.
- The secondary structure is formed by folding of polypeptide chain into series of beta (β) pleated sheets.
- The secondary structure is then folded into tertiary structure of compact globular domains.
- Finally these globular domains of adjacent heavy and light chain interacts in quaternary structure forming functional domains that enables binding site for antigen and the same time performs a number of biological functions.
- Two domains are found in L-chain ie one in variable region (VL) and other in constant region (CL).
- In H-chain, one domain is found in Variable region (VH). In IgA, IgG and IgD three domains are found in constant region (CH1, CH2 and CH3) whereas in IgE and IgM fou domains are found in constant region of H-chain (CH1, CH2, CH3 and CH4.
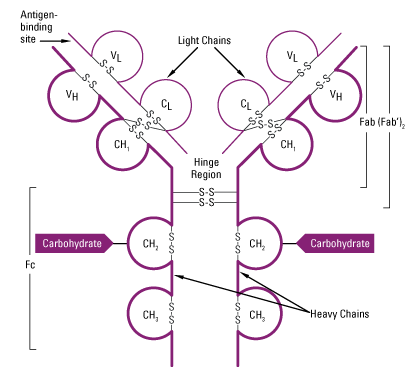
Fab, Fc and Hinge region of antibody:
1. Fab region
- Antigen binding is accomplished by amino-terminal (N-terminal) region and effector functions by carboxyl terminal (C-terminal) region of antibody.
- In an antibody molecule two Fab regions are found and they binds antigens.
- Hypervariable region on L-chain (VL domain) and H-chain (VH domain) form antigen binding site.
- Detailed comparison of aminoacids sequences of large number of VL and VH domain reveals that the sequence variation is concentrated in few discrete region of these domains. The variability plot of VH and VL domains shows maximum variation in certain region which is known as hypervariable region and this forms antigen binding site.
- Antigen binding site is complementary to epitope of antigen, so it is also known as complementary determining regions (CDRs).
2.Fc region:
- Fc region of immunoglobulin allows for interaction of immune complex with other phagocytic cells and complement.
- Take parts in various biological functions that are determined by aminoacid sequences of each domains of constant region.
- Many different form of Fc receptors exists.
3. Hinge region:
- The γ, δ and α heavy chain contains an extended peptide sequence between CH1 and CH2 domain that has no homology with other domain, this region is known as hinge region.
- Hinge region is rich in proline residue and is flexible. Therefore IgG, IgD and IgA are flexible.
- The flexibility given by hinge region enable Fab region to assume various angle to bind antigen.
Characteristics of antibodies;
- Antibodies are immunoglobulin (Ig) molecules
- Antibodies are antigen specific and binds to foreign molecules to host.
- They are produced by activated B-cells
- Antibodies are first molecules participating in specific immune response
- They mediate effector function to neutralize or eliminate foreign invaders.
Immunoglobulin classes or Isotypes
Antibody belongs to class of protein called Immunoglobulin (Ig). And classified into 5 classes or isotypes
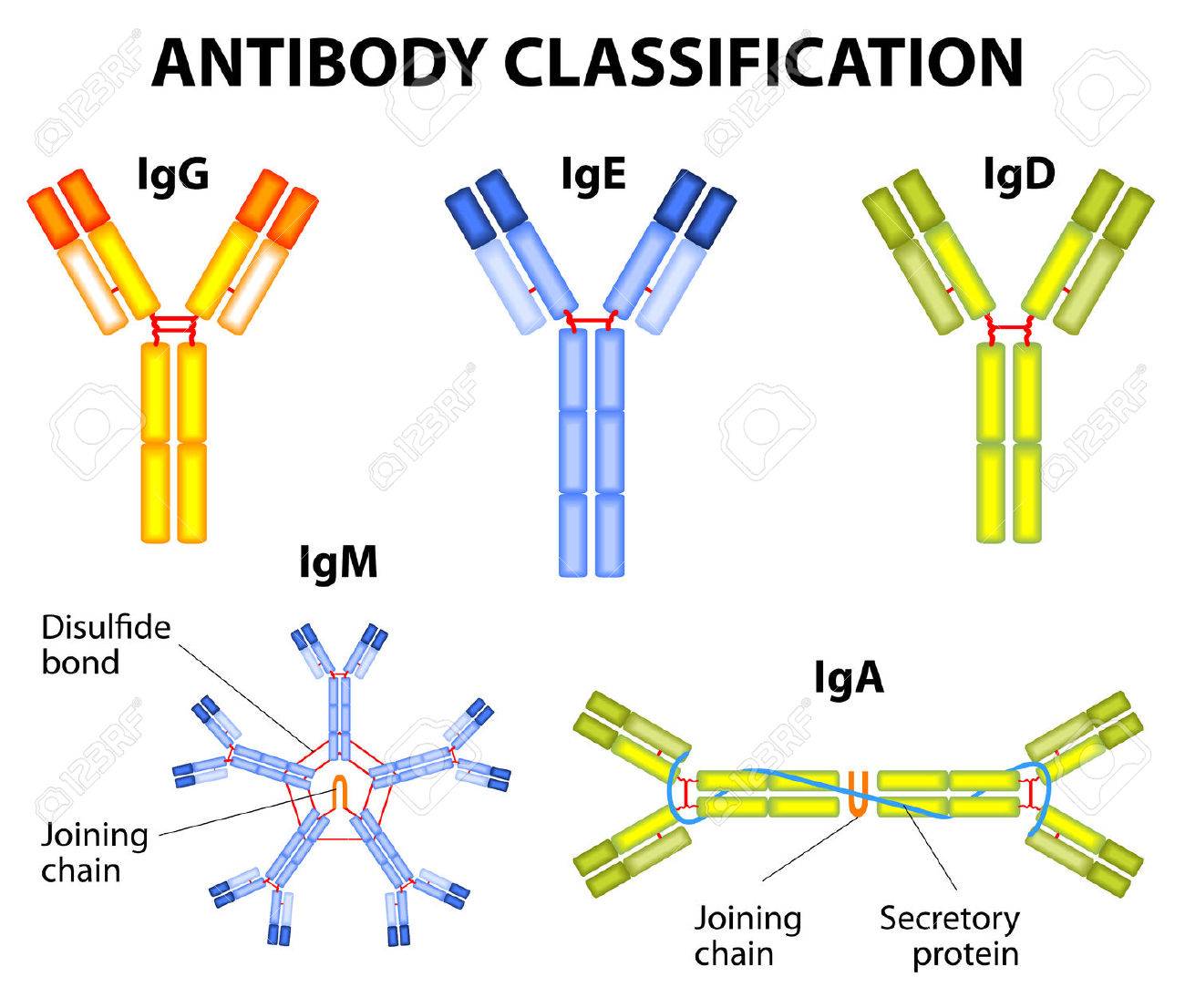
1. IgG:
- Molecular weight: 150,000 Da
- H-chain type: gamma (53,000 Da)
- IgG is the most abundant class of Immunoglobulin in serum and constitute of about 80% of total serum immunoglobulin.
- IgG molecule consists of two gamma (γ) heavy chains and two kappa (k) or two lambda (λ) light chains.
- There are four sub class of IgG ( IgG1, IgG2, IgG3 and IgG4) on the basis of decreasing serum concentration.
- It has longest half-life among other antibodies. Half-life is about 23 days.
- IgG is the only antibody that can cross placenta. It cross placenta and provide immunity to fetus upto 6 month of age. The immunity is known as natural passive immunity.
- It can also activate complement.
Biological functions:
- IgG is the major antibody produced in secondary immune response.
- Ig, IgG3 and IgG4 readily cross the placenta and play important role in protecting the fetus.
- IgG3 is the most effective complement activator followed by IgG1 and IgG2. IgG4 is not able to activate complement at all.
- IgG1 and IgG3 binds with high affinity to Fc receptor on phagocytic cell and thus mediate opsonization.
- IgG helps in bacterial immobilization.
- IgG neutralize toxin and viruses.
2. IgM:
- Molecular weight: 900,000 Da
- H-chain type: mu (65,000 Da)
- IgM accounts for 5-10% of total serum Immunoglobulin with an average serum concentration of 1.5mg/dl.
- IgM is secreted by plasma cell and it exists in pentameric form in which five IgM mononers are linked together by disulphide bond (J-chain).
- Due to large size, IgM is also known as millionare molecule.
- There are 10 antigen binding site (Fab) in pentameric IgM molecule but it cannot bind to 10 complete antigen due to steric hindrance.
- It is the major antibody produced during primary immune response.
- Monomeric form IgM (180000 Da) is also expressed as membrane bound receptor on B-cell.
Biological functions:
- IgM is the first antibody produced in primary immune response and it is also the first Ig produced by neonate.
- IgM has higher valency (antigen binding ste) due to its pentameric form.
- Due to pentameric form, IgG is very effective in agglutination reaction.
- IgM is more efficient than IgG in complement activation.
- IgM plays important accessory role as secretory immunoglobulin due to J-chain.
3. IgA:
- Molecular weight: 320,000 Da
- H- chain type: Alpha (55000 Da)
- IgA constitute 10-15% of total serum immunoglobulin.
- It is the predominant Immunoglobulin n external secretions such as breast milk, saliva, tears and mucus of bronchial, genitourinary and digestive tracts.
- IgA primarily exists as monomeric form but dimeric, trimeric and some tetrameric form are also present.
- IgA in blood occurs in monomeric form whereas those in body secretion occurs in dimeric or multimeric forms.
- Dimeric form of IgA contains J-chain and secretory chain. Secretory chains helps in transcytosis.
- IgA can cross epithelial layer and enter into body secretion. The process of crossing epithelial layer by IgA is known as transcytosis.
- There are two sub-class of IgA ie. IgA1 and IgA2.
Biological functions;
- IgA can cross the epithelial layer and enter into body secretion and provides local immunity in GI tracts, respiratory tract, genital tract etc
- In body secretion IgA neutralize viruses and prevent attachment on host surface.
4. IgD:
- Molecular weight: 180,000 Da
- H-chain type: Delta (70000 Da)
- IgD is present in extremely low concentration and it constitute 0.2% of total serum immunoglobulin.
- IgD together with IgM is the major membrane bound immunoglobulin expressed on mature B-cell.
- There are two sub-classes of IgD (IgD1 and IgD2)
- IgD plays important role in maturation and proliferation of B-cell.
5. IgE:
- Molecular weight: 200,000 Da
- H-chain type: epsilon (73,000Da)
- IgE accounts for 0.3% of total serum Immunoglobulin.
- IgE is also known as reagenic antibody due to its involvement in allergic reaction. IgE mediate immediate hypersensitivity reaction and responsible for symptoms like hey fever, asthma, anaphylactic shocks, etc.
- Fc region of IgE binds on blood basophils and tissue mast cells. The cross reaction with antigen to Fc region bound IgE causes degranulation of mast cell and basophils releasing histamine. Histamine is responsible for symptoms of allergy.
Biological functions;
- IgE provides immunity against parasite by Antibody dependent cell mediated cytotoxicity (ADCC).
- Level of IgE antibody in blood of normal individual is very low and its level increases during parasitic infection and in allergic reactions.
Antibody: Structure, classes and functions

Nice thanks