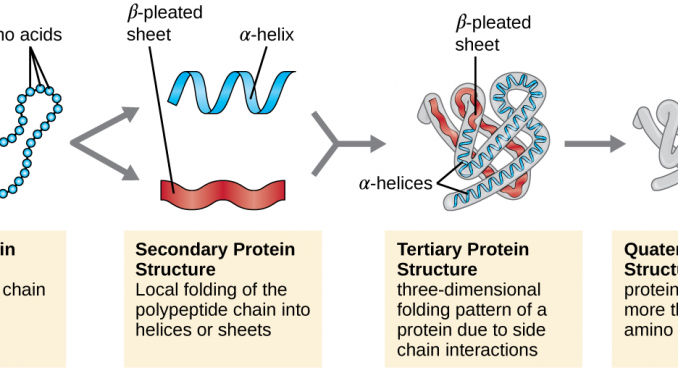
- Proteins are structurally organized into four level; primary structure, secondary structure tertiary structure and quaternary structure.
1. Primary structure of protein:
- Primary structure of protein refers to the sequence and arrangement of aminoacids in polypeptide chain.
- α-COOH group (carboxyl group) of one aminoacid is linked with α-NH2 group (amin0 group) of other aminoacid by peptide bond. The Peptide bond linked successive aminoacids in polypeptide chain.
- In polypeptide chain α-COOh group and α-NH2 group of most aminoacids are involved in formation of peptide bond. However, two aminoacids which are situated at either end of polypeptide chain have either –COOH group free or –NH2 group free.
- The end at which –COOH group is free is called C-terminal and other end at which –NH2 group is free is called N-terminal of polypeptide chain.
- Since, most of α-COOH and α-NH2 group formed peptide bond, they are not available for other bonding except Hydrogen bonding.
- The peptide bond occurs in TRANS-configuration and have partial double bond character.
- Due to partial double bond character between –CO and –NH group, they do not rotate during formation of folded secondary and tertiary structure. However, bonds between –Cα and –NH and –Cα and –CO rotate freely during folded structure formation.
2. Secondary structure of protein:
- Aminoacids that are located near to each other interacts to form regular arrangement called secondary structure.
- Formation of secondary structure involve local folding of polypeptide chain.
- There are three commonly occurring secondary structure. They are;
- α-Helix
- β-sheet
- β-bends or β-turn
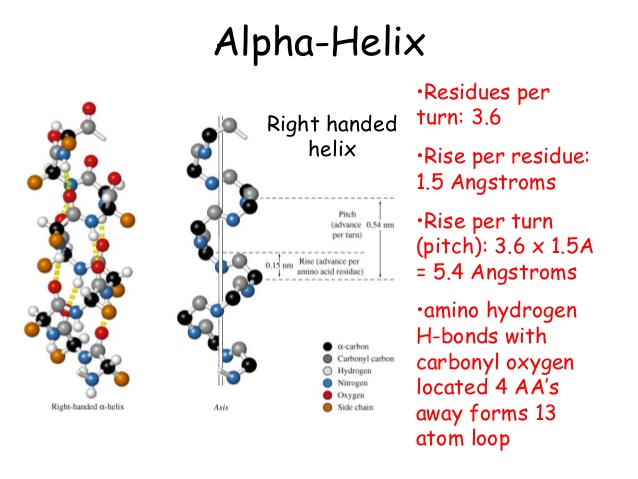
i. α-helix structure:
- α-helix is a right handed helical structure formed by twisting of polypeptide chain.
- It is a spiral structure.
- Each helix in α-helix structure contains 3.6 aminoacids residues. Vertical length of each helix is known as pitch which is 5.4 Å. Therefore, the vertical distance between two nearest aminoacids is 1.5Å which is called Identity period of α-helix.
- In α-helix, -C=O group of each aminoacid is hydrogen bonded with –NH group of other aminoacid which is situated four aminoacid ahead. Therefore, -C=O and –NH group of all aminoacids are hydrogen bonded in α-helical structure.
- R-group of aminoacid in α-helix are projected outward to minimize steric hindrance.
- Some aminoacids disrupt α-helix. For examples, the aminoacids with charged R-group disrupt α-helix by electrostatic repulsion or by formation of ionic bond. Similarly aminoacids with bulky R-group disrupt α-helix by steric interference.
- Aminoacids glycine and proline bring bend in polypeptide chain and disrupt α-ghelix.
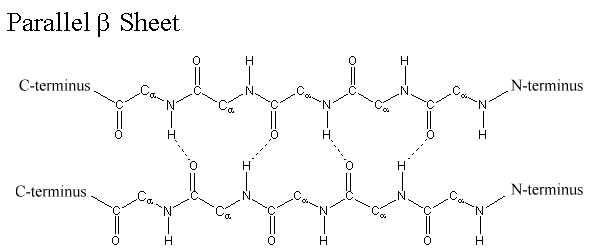
ii. β-sheet structure:
- β-Sheet is the most stable form of secondary structure of protein.
- It is formed between two different polypeptide chains which are placed parallel or antiparallel to each other.
- It can also be formed by folding of same polypeptide chain.
- In β-sheet structure, two polypeptide backbone are linked with each other by H-bond which are formed between –CO and –NH group.
- R-group of amino acids are alternately projected above and below the plane of β-sheet.
- The surface of β-sheet is not straight but it is pleated. Therefore, it is also known as β-pleated sheet.
β-sheet differ from α-helix in many characteristics as given below;
- Polypeptide backbone in β-sheet is extended rather than being tightly coiled as in α-helix.
- The axial distance between two nearest amino acid is 3.5 Å in contract with 1.5 Å in α-helix.
- In β-sheet hydrogen bond may be inter chain or intra chain but they are always inter chain in α-helix.
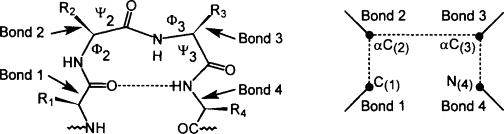
iii. β-bends or β-turn structure:
- β-bend reverse the direction of polypeptide chain and helps it to form globular (spherical ) structure.
- Β-bend structure consists of at least 4 amino acids in which nth amino acid is hydrogen bonded with (n+3)th amino acids.
- Glycine and proline are always found in β-bend structure.
- Ring of Proline attached with α-carbon atom helps to bend the chain. Similarly, lack of R-group in glycine permits great degree of rotation around α-carbon atom and bring bend in the polypeptide chain.
3. Tertiary structure of protein:
- Tertiary structure refers to the overall folding of a polypeptide chain to form a final three dimensional structure.
- For example, a globular protein which are larger than 200 amino acids units forms two or more domains by folding of polypeptide chain by either α-helix, or β-pleated sheet or β-bend. Finally these domains associates with each other to form final 3D structure.
- Therefore, tertiary structure refers to the formation of these domains by overall folding of polypeptide chain and then final association of these domains to form globular 3D structure.
- Bonds like H-bond, hydrophobic interaction, ionic bond and disulphate bond help in folding of polypeptide chain during formation of tertiary structure.
4. Quaternary structure of protein:
- Some proteins are composed of more than one polypeptide chain. Each polypeptide chain in such protein are called sub-units.
- The quaternary structure refers to interaction between these sub-units to form large final 3D structure. Therefore, quaternary structure is interaction between different polypeptide chains of multi chain protein.
- Quatarnary structure is found only in protein which are composed of more than one polypeptide chains such as hemoglobin
- Bonds like H-bond, ionic bond, hydrophobic interaction helps to from quaternary structure.
