
G-protein coupled receptors (GPCRs)
- G protein–coupled receptors (GPCRs) are receptors that are closely related with a member of the guanosine nucleotide–binding protein (G protein) family.
- The signal transduction through GPCRs are defined by three essential components:
- a plasma membrane receptor with seven transmembrane helical segments
- a G protein that circulates between active and inactive forms where active is GTP bound and inactive is GDP bound forms.
- an effector enzyme in the plasma membrane. It is regulated by activated G protein.
- When the G protein is stimulated by the activated receptor it exchanges bound GDP for GTP, then dissociates from the occupied receptor and binds to the nearby effector enzyme, altering its activity.
- The activated enzyme then produces a second messenger that influences downstream targets.
- The human genome encodes about 350 GPCRs for the detection of hormones, growth factors, and other endogenous ligands, and perhaps 500 that serve as olfactory (smell) and gustatory (taste) receptors.
- GPCRs have been employed in many common human disorders, including allergies, depression, blindness, diabetes, and different cardiovascular defects with serious health outcomes.
Adrenergic receptors
- Adrenergic receptors belongs to GPCRs family and there are four general types of adrenergic receptors, α1, α2, β1, and β 2 on the basis of differences in their affinities and responses to a group of agonists and antagonists.
- Adrenergic is another term for epinephrine, adrenaline.
- Agonists are structural analogs that bind to a receptor and mimic the effects of its natural ligand; antagonists are analogs that bind the receptor without triggering the normal effect and thus blocking the effects of agonists, including the biological ligand.
- The four types of adrenergic receptors are found in different target tissues and mediate different responses to epinephrine.
- The β -adrenergic receptors are present in muscle, liver, and adipose tissue. These receptors helps in signal transduction in case of change in metabolism such as the increased breakdown of glycogen and fat.
- The β- adrenergic receptor includes two sub types- β1, and β 2. however, their mechanism of action is same.
- As other GPCRs, the β -adrenergic receptor is an integral protein with seven hydrophobic, helical regions of 20 to 28 amino acid residues.
- These amino acid residues span the plasma membrane seven times, therefore the alternative name for GPCRs is heptahelical receptors.
Epinephrine signalling pathway through Beta-adernergic receptor
Step I: Binding of hormone to adrenergic receptor
- Epinephrine signals the need to flight or flee when some threat requires the organism to mobilize its energy-generating machinery.
- The action of epinephrine begins when the hormone binds to a protein receptor (adrenergic receptors) in the plasma membrane of an epinephrine- sensitive cell.
Step II: Interaction with G protein to activate effector protein (adenylyl cyclase):
- As soon as epinephrine binds to a site on the receptor deep within the plasma membrane, it triggers a conformational change in the receptor’s intracellular domain affecting its interaction with an associated G protein, aiding the dissociation of GDP and the binding of GTP .
- The G protein is heterotrimeric, composed of three different subunits: α, β, and γ.
- Such G proteins are therefore known as trimeric G proteins.
- In this case, it is the α- subunit that binds GDP or GTP and transmits the signal from the activated receptor to the effector protein.
- It is referred to as a stimulatory G protein, or Gs because this G protein activates its effector.
- Like other G proteins, Gs functions as a biological “switch”: when the nucleotide-binding site of Gs (on the α subunit) is occupied by GTP, Gs is turned ON and can activate its effector protein (adenylyl cyclase, in this case); with GDP bound to the site, Gs is switched OFF.
Step III: Dissociation of βγ dimer from Gs protein
- In the active form β, and γ subunits of Gs dissociate from the α, subunit as a βγ dimer, and Gsα, with its bound GTP, moves in the plane of the membrane from the receptor to a neighboring molecule of adenylyl cyclase.
- Gsα, is held to the membrane by a covalently attached palmitoyl group.
Step IV: Synthesis of cAMP
- Adenylyl cyclase is an integral protein of the plasma membrane, with its active site on the cytoplasmic face.
- The association of active Gsα, with adenylyl cyclase stimulates the the enzyme to catalyze the synthesis of cAMP from ATP raising the cytosolic level of cAMP.
- The interaction between Gsα, and adenylyl cyclase is possible only when Gsα, is bound to GTP.
- The stimulation by Gsα, is self-limiting; Gsα, has intrinsic GTPase activity that inactivates Gsα, by converting its bound GTP to GDP.
- Now the inactive Gsα, detaches from adenylyl cyclase, leaving cyclase inactive again.
- Gsα, re-associates with the βγ dimer (Gsβγ), and inactive Gs which is again available to interact with a hormone-bound receptor.
- The role of Gsα, in serving as a biological “switch” protein is not unique.
- A variety of G proteins act as binary switches in signalling systems with GPCRs and in many processes that involve membrane fusion or fission.
Step V: Activation of protein kinase A
- Epinephrine applies its downstream effects by the increase in [cAMP] that results from the activation of adenylyl cyclase.
- Cyclic AMP, in turn, allosterically activates cAMP-dependent protein kinase, also called protein kinase A or PKA which catalyzes the phosphorylation of specific Serine or Threonine residues of targeted proteins, including glycogen phosphorylase b kinase.
- The inactive form of PKA contains two identical catalytic sub-units (C) and two identical regulatory sub- units (R).
- The tetrameric R2C2 complex is catalytically inactive. It is because an autoinhibitory domain of each R subunit takes the substrate-binding cleft of each C subunit.
- When cAMP binds to the R subunits, they undergo a conformational change that moves the autoinhibitory domain of R out of the catalytic domain of C, and the R2C2 complex deforms to result two free, catalytically active C subunits.
- The allosteric activation of many types of protein kinases by their second messengers is mediated by the same basic mechanism- displacement of an autoinhibitory domain.
- The structure of the substrate-binding cleft in PKA is the prototype for all known protein kinases; certain residues in this cleft region have identical counterparts in all of the more than 1,000 known protein kinases.
- The ATP- binding site of each catalytic sub-unit positions ATP perfectly for the transfer of its terminal (γ) phosphoryl group to the -OH in the side chain of a Serine or Threonine residue of enzyme phosphorylase b kinase.
- The enzyme phosphorylase b kinase is active when phosphorylated and can begin the process of mobilizing glycogen stores in muscle and liver in anticipation of the need for energy, as signaled by epinephrine.
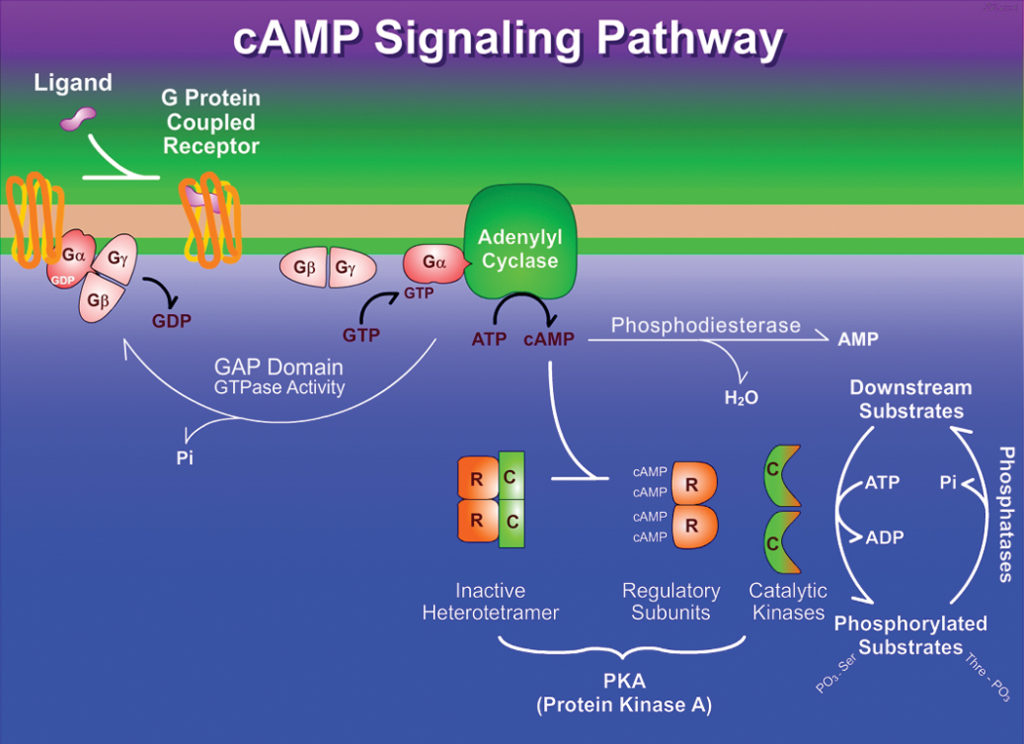
Fig: Transduction of the epinephrine signal: the beta-adrenergic pathway
