
Sex determination in human and role of various genes
- Sex determination is defined as the developmental decision that directs the bipotential gonad to develop as a testis or an ovary. In mammals, sex determination is genetically controlled depending on a developmental time and gene expression.
- Primarily sex determination is made at fertilization, but the embryo acquires its definite sex characteristics by a more complex mechanism called sex differentiation.
- Sex determination is not a single step method. But rather it involves various steps of sex differentiation.
- In human beings and other animals sex determination occurs in the following differentiation steps :
- Genetic Sex:
- Gonadal sex:
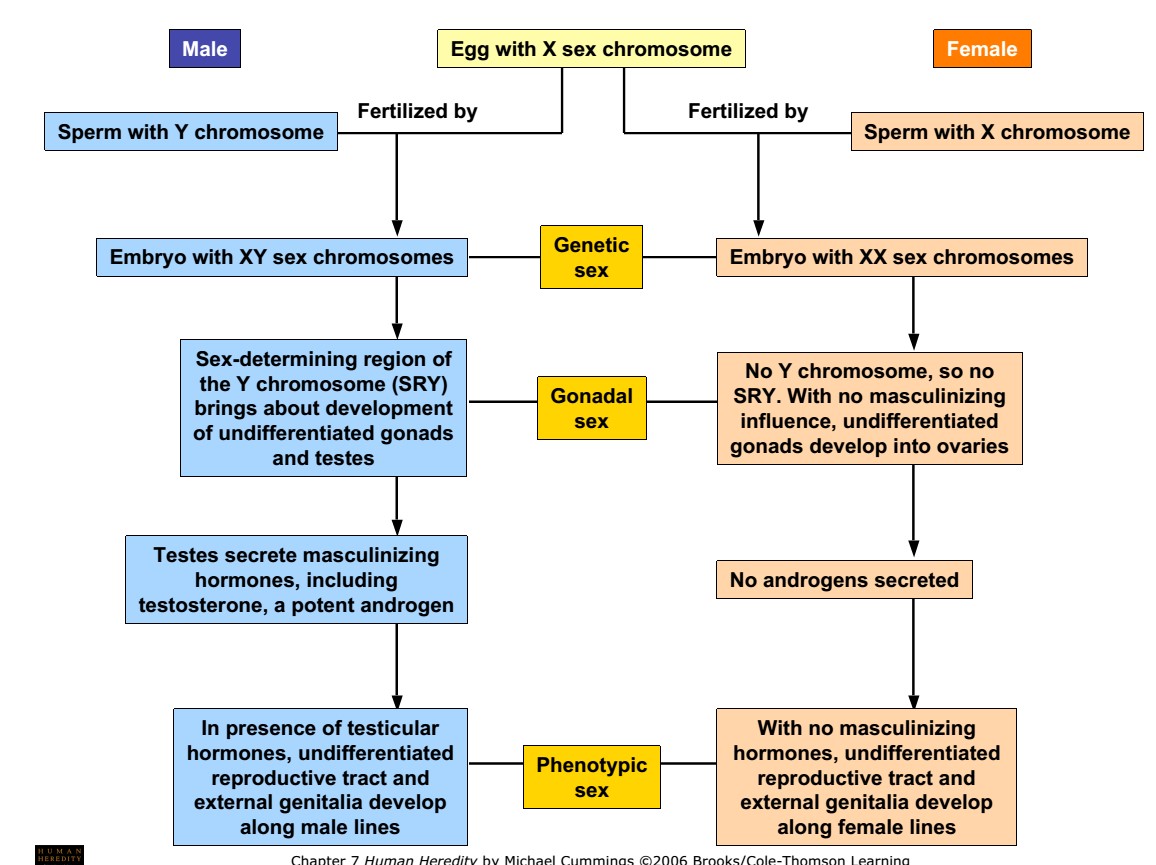
1. Genetic sex: chromosomal mechanism of sex determination
- Normal females have two X chromosomes and normal males have one X and one Y chromosome. Therefore, female is denoted as XX and male as XY.
- In Human as well as in other mammals, Y –chromosome controls sex.
- Conclusive evidence of this theory of Y-chromosome determining sex come from certain abnormal genetic syndromes such as Turner’s syndrome (XO Female; sterile female) and Klinefelter’s syndrome (XXY male; sterile male).
- In Turner’s syndrome, an individual is female despite having one X-chromosome and in Klinefelter’s syndrome, an individual is male despite having two X- chromosome.
- This evidence suggests that in human beings sex determination mechanism is Y- chromosome dependent like in plant (Melandrium) but not like Drosophila.
- There are genes on these sex chromosomes which determine femaleness or maleness.
Dosage Compensation:
- From the structure of X and Y chromosome, it was found that the X-chromosome carries much more genetic information than Y chromosome.
- Since females have two X- chromosome, they contain double dose of X-linked genes, whereas the male has only a single dose of these X-linked genes. This inequality in x-linked genes in female is tolerated by dose compensation mechanism.
- Dose compensation in human or mammal is achieved by inactivation of one X-chromosome in homogametic female (XX).
- In X-chromosome inactivation in mammals one X-chromosome gets characteristically condensed and inactivated. Such chromatin material is called facultative heterochromatin, since it becomes inactive in certain part of the life cycle and resumes activity before entering the germ line.
- The phenomenon of inactivation of X chromosome was confirmed by the observation of the Barr body.
- Barr and Bertram (1949) reported a deeply stained chromatin body (a chromocentre) in the nerve cells of female cat which was absent in the male. This chromatin body is called sex chromatin or Barr body after the name of its discoverer. Such Barr body has also been observed in most of the body cells (e.g., skin, oral epithelium and blood cells) of man and other mammals.
- Human females have the Barr body in the nuclei of their body cells in higher proportion than males and are, therefore, referred to as sex chromatin positive.
- The human males are sex chromatin negative.
- It has been demonstrated that the sex chromatin is derived only from one of the two X-chromosomes. The other X chromosome behaves like an autosome.
- Later, Lyon (1972) confirmed the existence of Barr body in normal females (XX), meta-females or super females (XXX) and in Klinefelter males (XXY).
2. Gonadal sex: transfer of Bipotential gonad into testis or ovary
- Until six weeks of foetal life, human embryo have identical undifferentiated gonads in male and female.
- Embryo before six weeks has two system of reproductive duct; wolffian duct and Mullerian duct.
- Wolffian duct: it develops into male reproductive tract
- Mullerian duct: it develops into female reproductive tract
- During this period of six weeks, the undifferentiated gonad have been invaded by the primary XX or XY cells.
- If gonad have been invaded by XY cells, the genes located in Y-chromosome called SRY gene causes the undifferentiated gonad to differentiate into a testis. Testis then begin producing two hormones; testosterone and anti-mullerian hormone.
- Anti-mullerian hormone (AMH) is mullerian inhibiting hormone which degenerates mullerian duct.
- Combination of events of these genes and other results in development of male phenotype.
- Testosterone and its other derivatives dihydro-testosterone induce formation of male sex organs and male reproductive duct.
- Similarly, if the gonad have been invaded by XX cells, the absence of SRY gene allows the gonad to become an ovary.
- Ovary begins producing estrogen hormone which induce development of uterus and cervix from mullerian duct.
- The development of gonad into a testis starts as soon as the gonocytes (primordial germ cells) from the yolk sac have finished their migration into the gonadal ridge.
- Gonocytes of the male (XY) migrate deeper into the gonadal blastema forming the medulla.
- Gonocytes of female gonocytes (XX) remain at the periphery of blastema, forming a thick cortical layer.
- Hence, the XX genotype develops into ovarian gonadal sex (female) and XY genotype develops into testicular gonadal sex (male)
Genes involved in sex differentiation in human:
#Role of Male sex-determining genes;
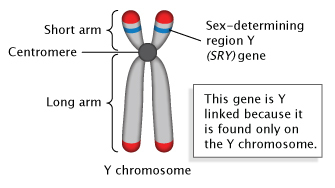
1. SRY gene:
- SRY gene of 35kpb on short arm of Y-chromosome known as sex determining region of Y (SRY) is the master regulator of sex determination.
- SRY is the segment of gene on Y –chromosome known as testis determining factor (TDF) in human. The TDF gene is the master switch that when turned on, activate on entire series of genes whose function is sex differentiation.
- In human or mammals, SRY encodes a transcriptional factor that activates testis formation after six weeks of foetal development.
- SRY gene activates other genes located in different chromosome and responsible for sex differentiation.
2. SOX9 gene:
- Sox9 is a member of the SOX transcription factor family which is located in autosome.
- SOX9 is induced by SRY gene.
- Immediately after expression of SRY gene, Sox9gene expresses in XY male gonad in lateral side and in sertoli cell precursors.
- Expression of SOX9 gene initiate differentiation of sertoli cell as well as production of testosterone and Anti-mullerian hormone (AHM) hormone. AHM causes degeneration of mullerian duct.
- Mutation in SOX9 gene results in loss of function and causes sex reversal from XY male to XY female as well as skeletal deformation.
- Therefore, SOX9 is responsible for sex determination as well as bone formation.
- Meanwhile, the duplication of SOX9gene in an SRY-negative female-to-male sex reversal patients demonstrated that SOX9 plays a crucial role in male sex determination and differentiation.
- Homozygous deletion of Sox9in developing mouse XY gonad leads to irregular sex cord formation, abnormal Sertoli cell differentiation, and reduced expression of male-specific makers
3. FGF9 gene:
- Fibroblast growth factor 9 (Fgf9) gene expression overlaps with SXO9 gene and results in early differentiation of sertoli cells.
- Loss of FGF9 gene results in male-to-female sex reversal.
- Therefore, FGF9 is also responsible for sex determination.
4. DMRT1 (Double sex and mab-3 related transcription factor 1) gene:
- Two copies of DMRT1 gene are present in human (Mammals) which are necessary for testis formation even in absence of SRY gene.
- DMRT1 gene are located in chromosome 9, so both sex (male and female) contains two copies of DMRT1 gene.
5. FOXL2 (Forkhead box L2):
- Recent studies have shown that two genes, doublesex and mab-3 related transcription factor 1 (Dmrt1) and forkhead box L2 (Foxl2), play critical roles in maintaining sexual phenotype in mice
6. Steroidogenic factor 1 (SF1 gene):
- SF1 gene is present in both sex and is required to form bipotential gonad from genital ridge.
- SF1 gene encode splicing factor 1 which is involved in production of steroid during embryogenesis. SF1 factor is also known as adrenal 4 binding protein which regulates number of other genes.
- SF1 gene is more activated in XY male than in XX female.
- Interaction of SF1 with SOX9 gene and acts on leydig cell to produce testosterone and also interacts with sertoli cell to produce AMH resulting in wolffian duct formation.
- While interaction of SF1 with DAX1 inhibit AMH and testosterone, resulting in mullerian duct formation.
#Role of female sex determining gene:
- XX females lack Y chromosome and SRY gene.
- Ovary formation for many years was considered to be a “default” gonad development pathway resulting from the absence of SRY
- Number of gene are responsible for ovary development. Such as-β-catenin, follistatin (Fst), FOXL2, R-spondin (RSPO1), and WNT4, without which ovary development cannot take place.
1. DAX1 (Dosage sensitive sex reversal) gene:
- DAX1 is encoded by NROB1 gene located on short arm of X-chromosome.
- Function of DAX1 is antagonistic to SRY, which means it is a negative modulator which decrease SF1 expression.
- DAX1 down regulate Anti-mullerian hormone leading to development of mullerian duct and ovary.
- Mutation on this gene results in reversal of XX female to XX male.
2. WNT4 gene:
- WNT4 is an autosomal gene present in chromosome 1.
- WNT4 is antagonistic to TDF preventing testis formation and it is required for ovary formation.
- Interaction of DAX1 with SF1 expresses WNT4 gene which is required for ovary formation.
- Mutation in WNT4 gene of XX female causes no ovary development and reversal of XX female into XX male.
