Leishmania donovani
- Leishmania donovani causes visceral leishmaniasis. The disease is also known as Dum-Dum fever, Asian fever, Assam fever or infantile splenomegaly in various parts of the world.
- The parasite is named after its discoverers-Leishman and Donovan. Both Leishman and Dobovan reported the parasite simultaneously in the same year, 1903.
- Leishman demonstrated the parasite in the spleen smear of a soldier in England, who died of fever contracted at Dum-Dum in Kolkata.
- Donovan found the same in the spleen smear of a patient suffering from kala-azar in India.
- The san fly (Phlebotomus argentipes) was identified as a vector of the disease by Indian Kala-azar commission (1931-1934).
Habitat:
- Leishmania donovani is an obligate intracellular parasite of man and other mammalian hosts.
- Promastigote forms of the parasite are found in sand fly and in culture
- Intracellular Amastigote forms are found in human in reticuloendothelial cells of the spleen, bone marrow, liver, intestinal mucosa and mesenteric lymph nodes.
Morphology:
- The parasite exists in two forms: Amastigote and Promastigote.
1. Amastigote:
- Amastogote is the aflagellar stage of the parasite.
- The parasite at Amastigote stage are found in man and other mammalian hosts.
- They are found inside monocytes, polymorphonuclear leucocytes or endothelial cells.
- Amastigotes are small, round to oval bodies measuring 2-3 µm in length.
- They are also known as LD (Leishman Donovan) bodies.
- Cell membrane is delicate and can be demonstrated only in fresh specimen.
- The nucleus is less than 1 µm in diameter, oval or round and is usually situated in the middle of cell.
- A rod –shaped kinetoplast lies at the right angles to the nucleus. It comprises of DNA containing body and a mitochondrial structure.
- Axoneme (rhizoplast) arises from the kinetoplast and extends to margin of the body. It represent the foot of the flagellum.
- Vacuole, which is clear unstained space, lies alongside the axoneme.
- They are stained well with Giemsa or Wright stain.
- In a Giemsa stained preparation the cytoplasm surrounded by a limiting membrane appear pale blue. The nucleus relatively is larger and stained red. The kinetoplast stained deep red.
- Amastigote divides by binary fission at 37°C.
2. Promastigote:
- Promastigotes are found in the digestive tract of sand fly (vector) and in the culture media.
- The fully developed promastigotes are long, slender and splindle-shaped. They measure 15 to 25 µm in length and 1.5 to 3.5 µm in breadth.
- A single nucleus is situated centrally.
- The kinetoplast lies transversely near the anterior end.
- The flagellum is single, delicate and measures 15- 28 µm and may be of same length as the body or even longer, projecting from front. The flagellum does not curve round the body of the parasite and therefore there is no undulating membrane.
- With Leishman stain, the cytoplasm appears blue, the nucleus pink or violet and the kinetoplast bright red.
- Promastigote multiplies by binary fission at 27°C.
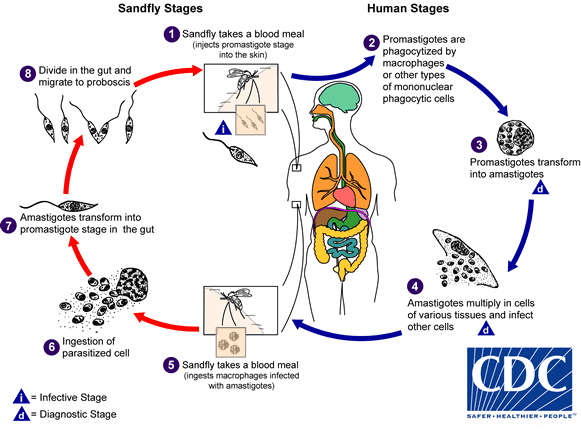
Life cycle of Leishmania donovani:
- The parasite has two stages in its life cycle:
- Amastigote form: occurring in humans and mammals.
- Promastigote form: occurring in sandfly.
- The parasite is transmitted to man or other vertebrate hosts by the bite of blood sucking female sand fly.
- During the blood meal, the sand fly deposits promastigote on the surface of the skin.
- These promastigotes are immediately phagocytized by macrophages and other types of mononuclear phagocytic cells. In these cells, promastigotes transform into the tissue stage of the parasite; amastigotes.
- The amastygotes multiply by simple binary fusion inside Reticuloendothelial system to form large number of amastigotes.
- The infected cell eventually becomes packed with the parasite.
- The host cell is thereby enlarged and ruptures.
- The parasites liberated infects new cells and the cycle is repeated and proceed to infect other mononuclear phagocytic cells.
- Some of the free amastigotes are phagocytosed by the neutrophils and macrophages in the blood stream.
- Free amastigotes are ingested by female sand fly during a blood meal from infected host.
- In the midgut of sand fly, the amastigotes are transformed within 72 hours through a series of flagellated intermediate promastigote forms to flagellated promastigotes.
- These promastigotes multiply by binary fusion and produce large numbers of promastigotes completely filling luman of the gut.
- After a period of 6-9 days, the promastigotes migrate from the midgut to the pharynx and buccal cavity of sand fly leading to heavy pharyngeal infection of the sand fly that feeds on plant juice after first blood meal.
- Bite of sandfly transmits the infection to new host and the life cycle is repeated.
Mode of transmission:
- The infection is transmitted to Human mainly by the bite of vector sandfly of genus Phlebotomus and genus Lutzomyia.
- Less frequently the infection is transmitte by:
- Blood transfusion, congenitial infection, accidental inoculation of cultured promastigotes in the lab workers and sexual intercourse.
- Males are affected more due to increase exposure through the occupation and leisure activities.
Pathogenesis of Leishmania donovani:
- After the inoculation of promastigotes by sand flies, they are deposited on the surface of skin and bind to macrophages in the skin.
- The sand fly, liberates biologically active substances, which promote infectivity of promastigotes by partially deactivating fixed macrophages in the skin.
- The outcome of leishmania infection appears to depend on the complex interaction between the parasite’s virulence and the immune response of the host.
- Promastigotes activate complement through the alternative pathway and are opsonized.
- They produce activated products of complement such as C3b or C3bi. These activated products bind with two specific receptors present on the outer membrane of promastigotes.
- The receptors are- a 63kD mol. wt glycoprotien (gp63) and a lipophosphoglycan (LPG). These receptors play an important role in the parasites- macrophage interaction.
- These receptors bind with complement receptors (CR3 and CR1) present on surface of macrophages either directly or through bound C3b or C3bi receptors.
- The most important immunological feature is a marked suppression of the CMI to leishmanial antigens. In persons with asymptomatic self-resolving infection, T-helper cells predominate, although immune suppression years later can result in disease. An overproduction of both specific Ig and non-specific Ig also occurs. The increase gamma globulin leads to reversal of the albumin-globulin ratio commonly associated with this disease.
- Leishmaniasis is a disease that involves the RE system. Parasitized macrophages disseminate the infection to all parts of body but more to the spleen, liver and bone marrow. The spleen is enlarged, with a thickening of the capsule and is soft and fragile, its vascular spaces are dilated and engorged with blood. The reticular cells are markedly increased and packed with the amastigote forms of the parasite. In the liver, the kupffer cells are increased in size and number and infected with amastigote forms. Bone marrow turns hyper plastic and parasitized macrophages replace the normal hemopoietic tissue.
- Proliferation and destruction of Reticuloendothelial cells of the internal organs and heavy parasitization of external organ by parasitized cells are the characteristic pathological changes seen in visceral leishmaniasis.
Clinical symptoms of Leishmania donovani:
- Visceral leishmaniasis (VL):
- Visceral Leishmaniasis (VL) also known as kala-azar, black fever and dum-dum fever is the most severe form of leishmaniasis.
- Pyrexia
- Fever
- Splenomegaly
- Hepatomegaly and Jaundice
- Lymphadenopathy
- Anemia
- Leucopenia
- Thrombocytopenia
- Skin lesions
- Hypergammaglobinemia
2. Post kala-azar Deremal Leishmaniasis (PKDL)
3. Cutaneous leishmaniasis (CL):
4. Lupiod leishmaniasis
5. Mocucutaneous leishmaniasis
Laboratory diagnosis of leishmaniasis:
a) Specimens:
- Splenic aspiration
- Bone marrow aspirations
- Lymph node aspiration
- Peripheral blood
b) Microscopy: smear preparation
- The amastigotes of Leishmania donovan is known as LD bodies
- LD bodies can be demonstrated in the smears of bone marrow, liver, lymph node and peripheral blood smear stained with leishman, giensa or eight stains.
- Brown Hopps staining is a recent method.
- LD bodies are seen within macrophages.
- Some of LD bodies can also be demonstrated free released from the cells ruptures during making of the smear.
c) Culture
- About 1-2 ml of blood (also splenic and bone marrow aspiration, other tissue and buffy coats of blood) is taken aseptically and diluted with 10ml of citrated saline solution.
- The cells are then either allowed to settle in a cool incubator (22°C) overnight or centrifuged.
- At the end of each week, a drop of condensation fluid is examined for promastigote forms.
- In a positive culture, motile promastigotes can be demonstrated microscopically in a few days to 4 weeks.
d) Blood count:
- Complete blood count shows leucopenia, normocytic normochromic anaemia and thrombocytopaenia.
- Total leucocyte count is decreased to 300/cc3.
- It shows relative lymphocytosis and monocytosis.
- Ratio of leucocytes to erythrocytes is altered as 1:2000 to 1:1000.
e) Napier’s Aldehyde test:
- This test depends upon an increase of gamma globulin in serum.
- In this test, a drop of 40% formalin is added to 1-2 ml of serum in test tube.
- A positive test shows jellification of a milky white opacity like the white of a hand boiled egg, within 2-20 minutes.
- Reaction occurring within 2 min is strongly positive.
- Disadvantage of test is that it shows false positive reaction with sera from the cases of schistosomiasis japonica, African trypanosomiasis, multiple myeloma and cirrhosis of liver.
f) Antimony test:
- This test also depends upon an increase of serum gamma globulin.
- A positive test is indicated by development of a white flocculent precipitate on addition of urea stilbamine solution to the patient serum.
- Not used nowadays.
g) Complement fixation test with W.K.K antigen:
- This test depends upon the presence of certain immune bodies in blood sera of kala-azar patients.
- The reaction is non-specific in character as the test is also positive in cases of lepromatous leprosy, PTB, tropical pulmonary eosinophila.
- Not used nowadays.
h) Detection of antibodies
- Various serological test have been developed to detect circulating specific antibodies for diagnosis of VL.
- This include immunoenzymatic technique, immunoblot, CIEF, THA, IFA, ELISA, Westem blot.
- Two serological test have been specifically developed for field used and have been sufficiently validated.
i) Direct Agglutination test (DAT)
- Widely used serological test for diagnosis of kala-azar
- Antigen used- trypsin treated, stained and formalin preserved promastigotes.
- Positive reaction – agglutination with specific antibodies from VL positive patient
- Test setting– room temperature
- The usefulness is limited by their variable sensitivity or specificity, requirement of electricity, refrigerator or a well-equipped lab and high cost.
j) The rk39 based immunochromatgraphic test (ICT):
- Rapid dip stick test
- Antigen used, recombinant k39 protien.
- K39 is an epitope apparently conserved on amastigotes of leishmenia spp
- Circulating anti-k39 gG is detectable in 95-100% patients who have kala-azar, irrespective of geographical region.
- Result can be obtained within 5 min.
- The test is found in LCT or dipstick format, that are more suitable for field case.
k) Animal inoculation:
- Wherever in-vitro facilitates are not there the materials from patients can be injected intraperitoneally in hamster or mice and the parasite is recovered from the animal.
- In positive cases, the amastigotes can be demonstrated in the stained impression smears of spleen from animal.
l) Leishmanin or Montenegro test:
- It is Delayed hypersensitivity test (DHT).
- 0.2 ml of leishmania antigen containing 6-10 millions of promastigotes per million is injected intradermally.
- The test result is read after 48-72 hours.
- Positive result is indicated by an induration of 5 mm or more.
- The test is positive in African kala-azar but not in India and Mediterranean kala-azar.
- The test is negative in untreated kala-azar and PKDL.
m) Alder’s test:
- It is a serological method.
- The development of promastigote forms of leishmania in Locke’s serum agar can be inhibited by an immune serum specific to L. donovani, L.tropica and L. brasiliensis.
n) Live function test (LFT):
- LFT show mild elevation in the levels of ALP, aspartate aminotransferase, alanine aminotransferase.
o) Molecular diagnosis
- DNA probes
- PCR.
Treatment of Leishmaniasis:
- Penta-valent antimonials:
- Meglumine antimonate
- Sodium stibogluconate solution
- MOA: interfere metabolism of the parasite.
- Dose: 20 mg antimony base/kg/day to 850 mg for at least 20 days for adults and 30 days for infants.
- Others drugs : Pentamidine, Amphoterisin B, Miltefosine, Interferon
Prevention and Control of Leishmania donovani:
- Reservoir control
- Active and passive case detection
- Treatment of those found infected including PKDL
- Killing of infected dogs in case of zoonotic kala-azar
- Vector control
- Reduction of sand fly population by insecticides mainly DDT, dieldrin, malathion.
- Concomitantly prevent VL and other vector borne disease, such as malaria and JE
- Health education to community about cause, MOT of leishmaniasis
- Using insect repellent, bed nets and window mess
- Keeping environment clean