
Enzymes
Enzymes are biological catalyst that catalyze biochemical reaction during metabolism but itself remain unaffected during the process of catalysis. Enzymes accelerate the rate of biochemical reaction by decreasing the energy of activation. Almost all the enzymes are protein in nature except ribozymes.
Properties of enzymes:
- Chemical nature:
- All enzymes are protein in nature except ribozyme. Ribozyme is an RNA molecule
2. Active site:
- All enzymes molecules contain a special cleft or pocket in its structure which is actively involved in catalysis. This cleft or pocket is known as active site of enzyme.
- Active site binds with substrate and the functional group present in active site modify the substrate during catalysis.
3. Specificity:
- Enzyme catalyzed reaction are highly specific ie. A particular enzyme catalyze particular type of biochemical reaction.
- There are two types of enzyme specificity. One is absolute specificity and other is group specificity.
- i) Absolute specificity: if enzyme acts on only one type of substrate, it is called absolute specificity. For example, catalase enzyme acts on H2O2 only. Similarly, Urease enzyme acts urea only.
- ii) Group specificity: some enzymes acts on group of closely related substrates. This type of specificity is known as group specificity. For example, enzyme Alcohol dehydrogenase bring dehydrogenation of many alcohols such as methanol, ethanol etc. similarly, enzyme hexokinase phosphorylates many hexose sugar including, glucose, galactose, mannose etc.
4. Catalytic efficiency:
- Enzyme catalyzed reaction are highly efficient.
- Rate of enzyme catalyzed reaction is usually (10^3- 10^8) times faster than uncatalysed reaction.
- Efficiency of enzyme is expressed in turn over number. Turn over number is defined as number of substrate molecule converted into product by one enzyme molecule in one second.
5. Co-factor and co-enzyme:
- Some enzyme are composed of protein part only (eg. Pepsin) whereas other enzymes are composed of both protein part and non-protein part.
- The complete enzyme molecule (with both protein part and non-protein part) is known as holo-enzyme.
- The protein part is known as Apo-enzyme
- Non- protein part may be co-factor or co-enzyme
- Co-enzyme is organic molecules such as NAD+, NADP, FAD+ etc present with Apo-enzyme.
6. Enzyme regulation:
- Enzymes are regularly regulated in cell or body depending upon need.
- Enzyme synthesis can be activated or inhibited upon need in cell.
Mechanism of enzyme action
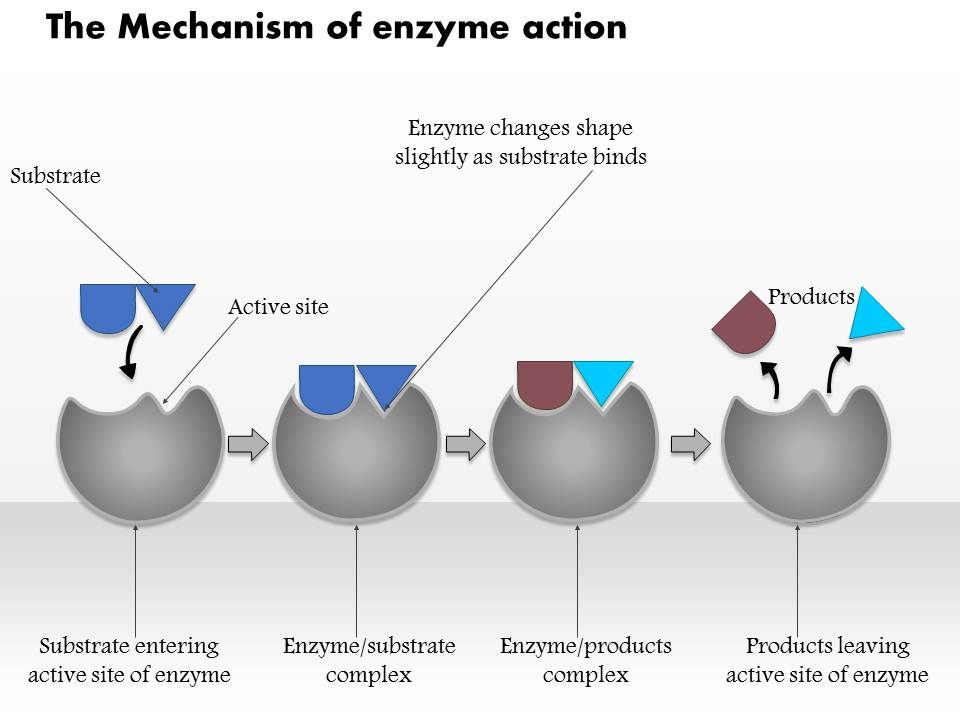
Enzymes are very specific and it was suggested by Fischer in 1890 that this was because the enzyme had a particular shape into which the substrate or substrates fit exactly.
This is often referred as Lock and Key hypothesis.
I. Lock and Key model:
- According to this model, shape of active site of enzyme is complementary to the shape of substrate molecules. Ie. the substrate is like a key whose shape is complementary to the enzyme which is supposed to be lock and they fit perfectly.
- Enzymes catalyze only those substrates which fit perfectly on the active site of that enzyme.
- Most enzymes are far larger than the substrates molecules that act on and the active site is usually a very small portion of the enzyme, between 3 and 12 amino acids. The remaining amino acids which make the bulk of the enzyme, function to maintain the correct globular shape of the enzyme.
- Once the product is formed, they no longer fit into the active site and escape into surrounding medium.
- According to lock and key model, enzymes behave as rigid molecules. However, most enzymes are globular and are flexible with varying shape.
II. Induced fit model:
- In 1959, Koshland suggested a modification to the ‘Lock and Key’ hypothesis which is known as ‘Induced fit’ hypothesis.
- Working from evidence that suggested that some enzymes and their active site are more flexible. To this, he proposed that the active site can modify its shape as the substrate interact with the enzyme.
- The amino acids which make up the active site are moulded into precise shape which enable the enzyme to perform its catalytic function most efficiently.
- For instance, a suitable analogy to describe Induced fit model would be that of a hand changing the shape of the glove as the individual put on the glove. Therefore in this case, glove is the active site of enzyme and the hand is substrate.
- However, in some cases, the substrate molecules changes slightly as it enters the active site before binding.
How enzyme catalyze the reaction?
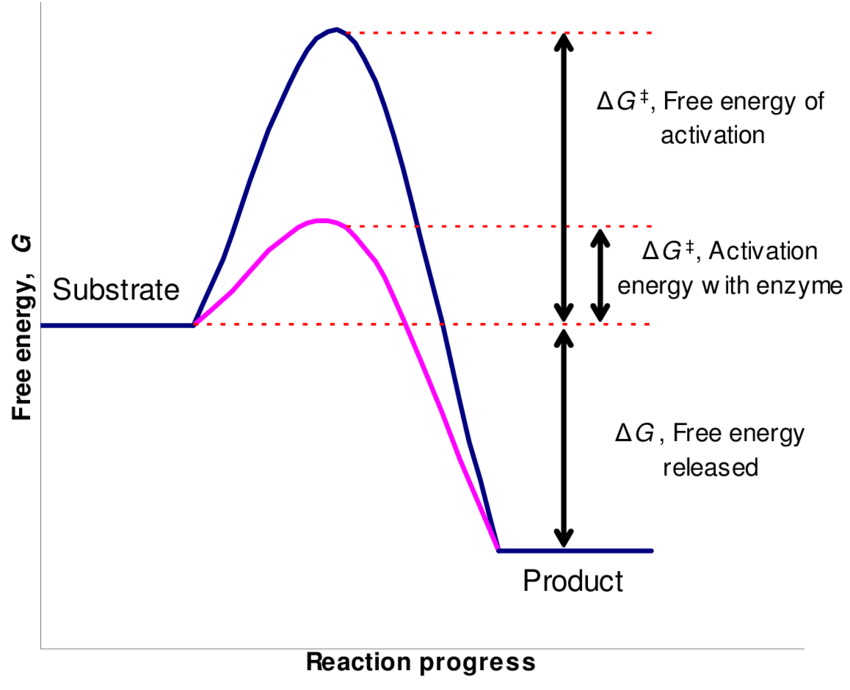
- Enzymes are catalyst that accelerate the rate of biochemical reaction by decreasing the energy of activation.
- Every chemical reaction have energy barrier that must be crossed by the reactant molecules in order to convert itself into the product.
- The amount of energy supplied to reactant molecules in order to cross the energy barrier to from product is known as Energy of activation.
- If energy of activation is higher, rate of reaction is slower and if it is lower, the rate of reaction is faster.
- The role of enzyme in biochemical reaction is to reduce the amount of energy of activation such that the rate of reaction increases.
- During enzyme catalysis, active site of enzyme binds with substrate molecules to form Enzyme-substrate (ES) complex. During this binding some binding energy is released which is utilized to activate the substrate (reactant) molecules to form product. Thus the requirement of the amount of activation energy is decreased such that rate of reaction increases. The amount of activation decrease is equal to the amount of binding energy released during binding of enzyme and substrate.
