Cholesterol
- Cholesterol is a steroid alcohol present in animal tissue. It is the structural component of all cell membranes and regulating fluidity. It is also the precursors of bile acids, steroid hormones and vitamin D.
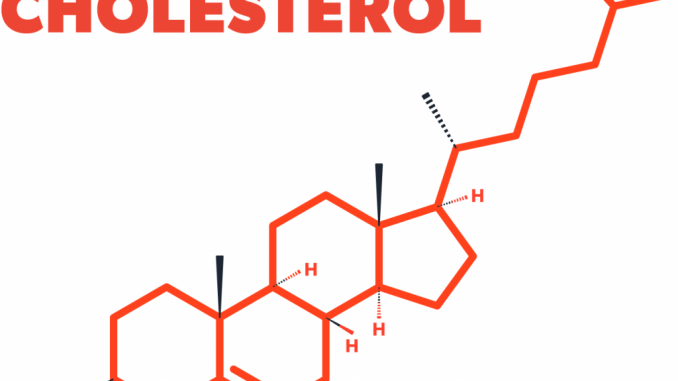
Structure of cholesterol:
- Cholesterol is a hydrophobic compound.
- It consists of four fused hydrocarbon rings (A, B, C and D called steroid nucleus) and it has 8 carbon branched hydrocarbon chain attached to C17 of D-ring.
- Ring-A has a hydroxyl group at C3
- Ring-B has a double bond between C5 and C6
Biosynthesis of cholesterol:
- Cholesterol is synthesized by virtually all tissues in human, although liver, intestine, adrenal cortex, reproductive organs (testis and ovaries) and placenta make largest contributions to the body’s cholesterol pool.
- Cholesterol is an essential molecules in many animals, including human but is not required in diet as all cells can synthesize it from simple precursors.
- Cholesterol is 27 carbon containing compound. All the carbon atoms in the cholesterol is provided by acetate. NADPH provides the reducing equivalents.
- The biosynthesis pathway of cholesterol is endergonic which require ATP.
- For the production of 1 mole of cholesterol, 18 moles of Acetyl coA, 36 moles of ATP and 16 moles of NADPH are required.
Steps of cholesterol biosynthesis:
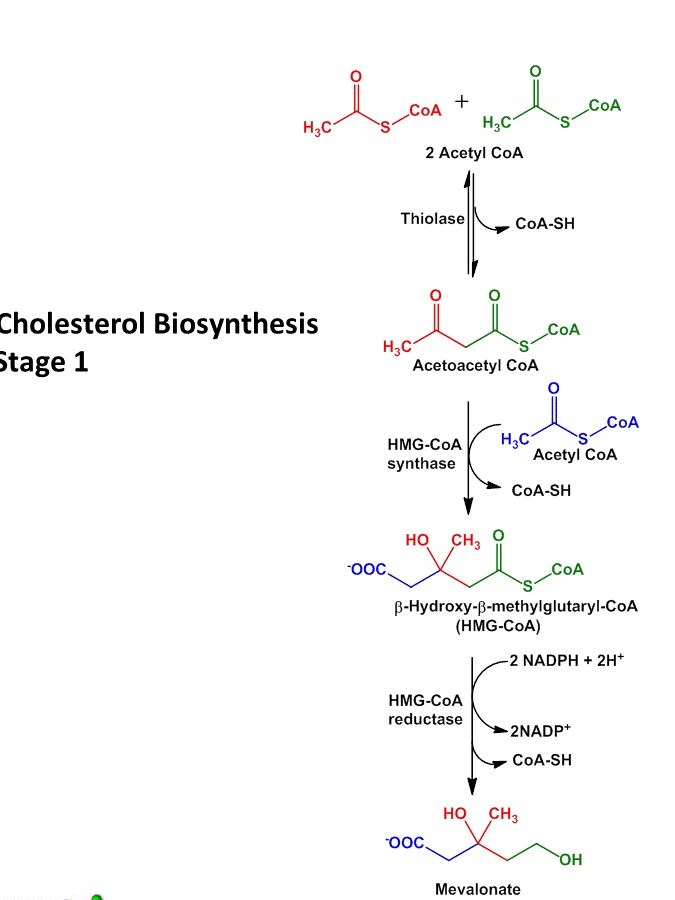
Step I: synthesis of HMG-coA (β-hydroxy-β-methyl-glutaryl coA)
- The first step in cholesterol biosynthesis is similar as ketone body formation.
- Two molecules of acetylcoA condenses to form AcetoacetylcoA. The reaction is catalyzed by enzyme thiolase.
- AcetoacetylcoA condenses with another molecule of acetylcoA to from β-hydroxyl-β-methyl-glutaryl-coA (HMG). This reaction is catalyzed by HMG-coA synthase.
- The cytosolic enzyme HMG-coA synthase participates in cholesterol synthesis whereas mitochondrial HMG-coA synthase participates in ketone body synthesis.
Step II: Reduction of HMG-coA into Mevalonate
- Reduction of HMG-coA to Mevalonate is catalyzed by HMG-coA reductase enzyme.
- It is the rate limiting step in cholesterol biosynthesis and also it is the key step in regulation of cholesterol biosynthesis.
- The enzyme HMG-coA reductase is present is cytosol and catalyze the reduction of HMG-coA into Mevalonate in which 2 NADPH are used up for donation of 2 electrons.
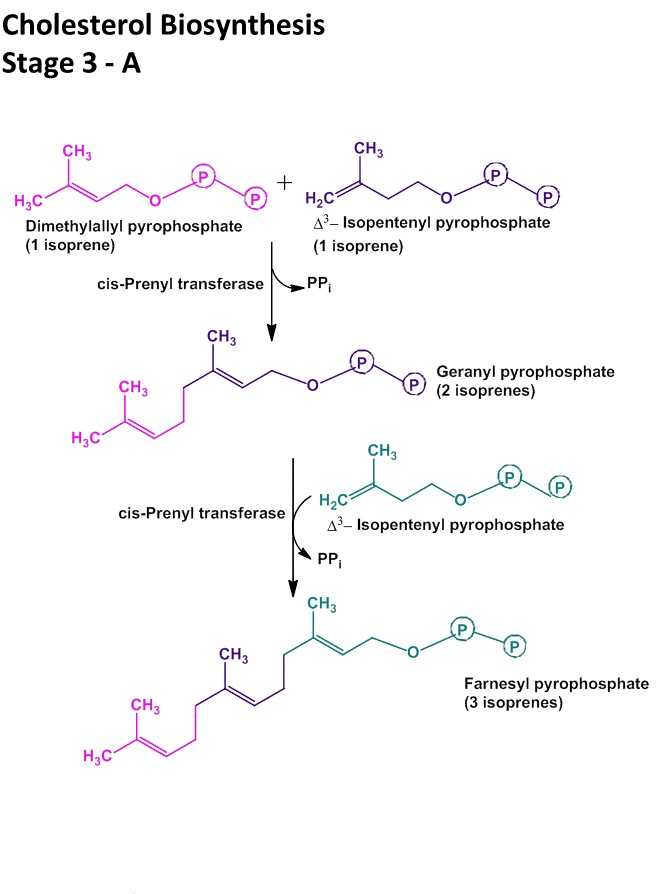
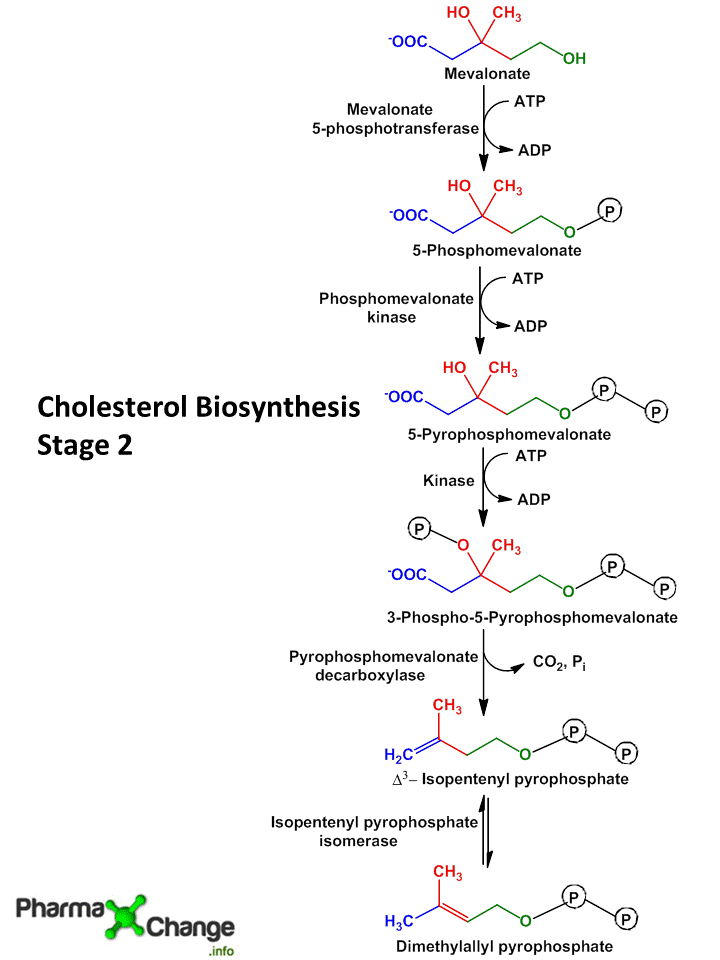
Step III: Conversion of Mevalonate to two activated Isoprene units
- In this step three phosphate groups are transferred from three ATP molecules to Mevalonate.
- The phosphate group attached to C3 hydroxyl group of mevalonate in the intermediate 3-phospho-5-pyrophospho-mevalonate leaves which is followed by decarboxylation producing a double bound in C5 and the product is known as isopentyl-pyrophosphate (IPP)
- Isomerization of isopentyl-pyrophosphate (IPP) yields 3,3- dimethyl-allyl-pyrophosphate (DPP).
- Both IPP and DPP are 5 carbon isopyrene unit.
Step IV: condensation of six activated Isoprene units to form Squalene
- Isopentylpyrophosphate and dimethylallylpyrophosphate now undergoes head to tail condensation in which one pyrophosphate is displaced and 10-carbon Geranylpyrophosphate (GPP) is formed.
- GPP undergoes another head to tail condensation with isopentylpyrophosphate yielding 15-carbon intermediate Farnesylpyrophosphate (FPP).
- Finally two FPP molecules joins head to head with elimination of both pyrophosphate group to form Squalene.
Step V: conversion of Squalene to Lanosterol (a four ring steroid nucleus)
- Squalene undergoes hydroxylation and cyclization utilizing oxygen and NADPH and get converted to lanosterol, which contains four ring steroid nucleus
Step VI: conversion of lanosterol into cholesterol
- Lanosterol undergoes series of about 20 reactions to finally convert into cholesterol.