Principle
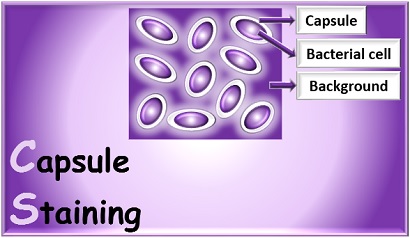
A capsule is non-ionic and very delicate structure that is secreted by the cell and that surrounds and adheres to the cell wall. It is present in few bacteria. Bacteria having thick capsule outside their cell wall are generally virulent and capable of causing disease, since this gelatinous structure protects bacteria against the normal phagocytic activities of host cells. The capsular material is composed mainly of complex polysaccharides such as levans, dextrans, and celluloses.
Due to non-ionic property of capsule, its staining is more difficult than other types of differential staining procedures and again the capsular materials are water-soluble and may be dislodged and removed with vigorous washing. During capsule staining procedure, smears should not be heated because the resultant cell shrinkage may create a clear zone around the organism that is an artifact that can be mistaken for the capsule.
The capsule stain uses two reagents.
Primary Stain: 1% aqueous solution of Crystal Violet is used as primary stain. It is applied to a non–heat-fixed smear. Both the cell and the capsular material will take on the dark color.
Decolorizing Agent: In the capsule staining, 20% Copper Sulfate (20%) is used as a decolorizing agent rather than water. Since the capsule is non-ionic, the primary stain adheres to the capsule but does not bind to it. The copper sulfate solution wash out the primary stain from the capsular material without removing the stain bound to the cell wall. And now the decolorized capsule absorbs the copper sulfate, acquires its blue color and will now appear blue in contrast to the deep purple color of the cell wall.
Requirements:
- Cultures: Skimmed milk cultured for 48 hours with Leuconostoc mesenteroides, and Klebsiella aerogenes
- Reagents: 1% crystal violet and 20% copper sulfate (CuSO4.5H2O)
- Equipment: Micro-incinerator or Bunsen burner, inoculating loop or needle, staining tray, bibulous paper, lens paper, glass slides, and microscope.
Procedure for capsule staining
Capsule staining is an example of negative staining, however in this procedure, we did not stain the background with acidic stain. Only basic stain (crystal violet) is being used to stain the bacterial cell wall.
- Take a clean grease free glass slide.
- Place several drops of crystal violet stain on the slide.
- Using aseptic technique, add three loopfuls of a culture to the stain and prepare a smear by gently mixing with the inoculating loop.
- With another clean glass slide, spread the mixture over the entire surface of the slide to create a very thin smear.
- Let the slide stand for 5 to 7 minutes.
- Allow the smears to air-dry. (but do not heat fix)
- Wash smears with 20% copper sulfate solution.
- Gently blot dry and examine under oil immersion.
- Repeat Steps 1–5 for each of the remaining test cultures
- Record the observations; Indicate the color of the capsule and of the
cell on each preparation
Result interpretation:
- The capsule appears as blue colored between deep purple background and cell wall.
- Leuconostoc mesenteroides, and Klebsiella aerogenes= capsule present
List of some capsulated bacteria:
- Gram negative and capsulated: Neisseria meningitides, Klebsiella pneumoniae, Haemophilus influenza type B, Pseudomonas aeruginosa, Escherichia coli (few strains), Yersania pestis,
- Gram positive and capsulated: Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogens, Bacillus megaterium, Bacillus anthracis,
- Capsulated yeast: Cryptococcus neoformans
