Principle:
This test was first described by Christie, Atkins and Munch-Petersen (1944) and the test name CAMP is derived from the initials of the author’s name. It was found that Streptococcus agalactiae produce a diffusible, extracelluar and heat stable protein called CAMP factor that acts synergistically with the beta-hemolysin produced by Staphylococcus aureus, such that the effect is a zone of enhanced lysis on sheep or bovine blood agar. It is a test used for the presumptive identification of group B streptococci (Some group A streptococci may also give a positive test, particularly under anaerobic conditions). The organism under test is inoculated in a fine streak on the surface of bovine or sheep-blood agar; a second streak – perpendicular to the first but separated from it by a few millimetres, is made with a culture of beta-hemolysin producing strain of Staphylococcus aureus. The plate is then incubated (aerobically) for about 12 hours or more at 37°. In a positive test, a typical arrowhead or flame-shaped area of clear haemolysis – due to synergy between a streptococcal CAMP factor (an extracellular polypeptide) and staphylococcal beta- hemolysin occurs between the two lines of bacterial growth. The CAMP test is used for the detection of Streptococcus agalactiae (a causative agent of bovine Mastitis) in milk. A reverse CAMP test, using a known group B streptococci (S. agalactiae) can be used for the identification of b-haemolysin-producing staphylococci (S. aureus).
Requirements:
- Blood agar
- Beta-lysin reagent
- Culture of S. aureus ATCC 25923
- Positive control- culture of S. agalactiae ATCC 12382
- Negative control _ culture of Streptococcus pyogenes ATCC 19615
- Disks containing beta-lysin of S. aureus
- Spot CAMP liquid reagent
Preparation of spot CAMP reagent for rapid CAMP test
- Inoculate two 5-ml tubes of Brain heart infusion (BHI) agar with a swab of growth from a fresh subculture of S. aureus ATCC 25923.
- Incubate overnight (shaking is optional) at 35° C in air.
- Working with gloves in a laminar-flow safety cabinet, combine the two broth suspensions and filter sterilize them using a 0.45 µm-pore-size cellulose-acetate filter.
- Aliquot the filter-sterilized broth into small tubes of about 1 ml.
- Label with the name of reagent (CAMP), date of preparation, and expiration date of 6 months from preparation.
- Freeze at 20°C or lower.
- Store defrosted reagent at 4°C and use within 2 weeks. Do not refreeze.
Procedure
Standard CAMP test:
- Streak S. aureus ATCC 25923 in a straight line across the center of the Blood Agar plate.
- Streak the unknown microorganism in the same manner perpendicular to the Staphylococcus, but avoid touching the previously streaked area.
- Streak the positive control organism parallel to and approximately 1 inch from the unknown organism.
- Label the location of each streak on the back of the plate.
- Incubate the plate overnight at 35° C in a CO2 incubator.
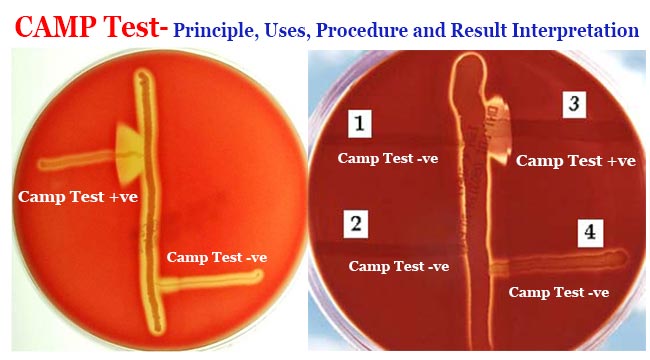
- Result interpretation:
- In standard CAMP test, a positive result is indicated by the formation of a distinct arrowhead of hemolysis at the intersection of the streaks of staphylococcus and test organism.
- In reverse CAMP, a positive result is indicated by a distinct arrow of no hemolysis at the intersection of the two hemolytic organisms.
CAMP test by Disk method
- Place disk on warmed Blood agar plate.
- Streak microorganism 2 to 3 mm from the edge of the disk.
- Incubate the plate overnight at 35° C in a CO2 incubator.
- Result interpretation
- In the disk test, a positive result is indicated by a distinct crescent or arc-shaped
- zone of complete hemolysis at the intersection of the disk of beta-lysin and the isolate.
Rapid CAMP test:
- Place 1 drop or a 10 µl of CAMP reagent next to a presumptive S. agalactiae colony growing on Blood agar. (Do not worry if the liquid touches or even engulfs the colony).
- Incubate the plate right side up, to prevent the spot CAMP reagent from running over the plate’s surface, for 20 min at 35°C.
- Examine with transmitted light for a zone of enhanced hemolysis next to the colony.
- Reincubate for up to 30 min if reaction is initially negative.
- Use a hand lens if necessary for examining the plate.
- Refrigeration may enhance reaction after incubation.
- Result interpretation:
- In the rapid spot test, positive result is indicated by the presence of clear enhanced hemolysis only where the diffused hemolysis overlapped.
- Lack of enhanced hemolysis near the colony being tested is a negative test
Organism giving CAMP test positive:
- A streptococcus which gives a positive CAMP test and is morphologically and biochemically consistent (catalase-negative, gram-positive cocci in pairs and chains) is reported as Streptococcus agalactiae.
- List of Gram-positive rods that give CAMP test positive:
- Rhodococcus equi
- Listeria monocytogenes
- Propionibacterium avidum/granulosum,
- Actinomyces neuii
- Turicella otitidis
- Corynebacterium glucuronolyticum
- Corynebacterium colyeae
- Corynebacterium imitans, and some strains of Corynebacterium striatum and Corynebacterium afermentans group.
