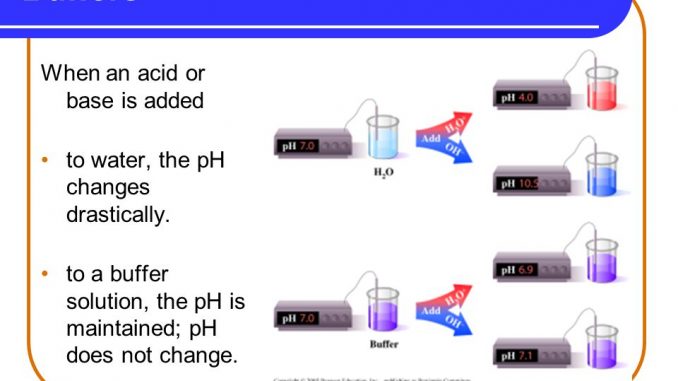
Buffer:
- Buffers are defined as solutions which resists small change in pH by adding small amount of acid or base.
- A buffer usually consists of a weak acid and its salt (fore eg, acetic acid and sodium acetate) or a weak base and its salt (for eg, ammonium hydroxide and ammonium chloride).
Mechanism of buffer action:
CH3COOH ß——à CH3COO- + H+
CH3COONa ß—-à CH3COO- + Na+
- When HCL is added H+ ion combines with CH3COO- and resists change in pH. Similarly when a base (NaOH) is added H+ ion and OH- ion combines to form H2o which also rsist pH change. Hence the buffer solution resist change in pH when adding small amount of acid or base.
Buffering capacity:
- The efficiency of a buffer in maintaining a constant pH on addition of acid or base is referred to as buffering capacity. It mostly depends on the concentration of buffering component. The maximum buffering capacity is achieved by keeping same concentration of acid and its salt or base and its salt.
Properties of good buffer:
- The buffer should be non-toxic
- The buffer should be able to penetrate cell membrane and should not absorbs light at UV or visible region
- Buffer should have adequate buffering capacity
- Buffer should not form insoluble complex with any anions or cations in the reaction.
Role of buffer in vitro:
- Acid-base buffer helps in tissue or organ preservation ( eg phosphate buffer)
- In tissue culture, the optimum pH is maintained by buffers. For example HEPES buffer is widely used in cell culture because it is better at maintaining physiological pH despite change in CO2 concentration in media.
- HEPES also maintains enzyme structure and function at low temperature
- Buffer are used in fermentation process
- It is also used for setting correct condition for dyes used in coloring fabrics
- Buffer is used to calibrate pH meter
Role of buffer in vivo
There are three buffer system in human body to maintain acid-balance.
- Bicarbonate buffer
- Phosphate buffer
- Protein buffer
Bicarbonate buffer:
- Sodium bicarbonate and carbonic acid constitute bicarbonate buffer. It is the most predominant buffer system in plasma.
- It is extracellular buffer
- It helps in maintaining blood pH 7.4
Phosphate buffer:
- Sodium dihydrogen phosphate and disodium hydrogen phosphate constitute phosphate buffer.
- It is intracellular buffer
Protein buffer:
- The plasma protein and hemoglobin together constitute protein buffer system of blood.
- Th ebuffering capacity of protein buffer depends upon pKa of amino acids.
- The imidazole group of histidine (pKa= 6.7) is the most effective contributor of protein buffer.
- Hemoglobin of RBCs is also an important buffer. Hb binds to H+ ions and helps to transport CO2 as HCo3- ion with minimum change in pH. In lungs Hb combines with O2 then H+ ion is released and combine with HCO3- to form carbonic acid (HCO3-). Later carbonic acid dissociates to release CO2.
