B-cell development:
- The development of plasma cell and memory B cells can be divided into three broad stages:
- Generation of mature, immunocompetent B-cells (maturation)
- Activation of mature B-cells and the differentiation of the activated B-cells, into plasma cells and memory B cells.
- These three stages can be divided into two phases:
- Antigen independent phase:
- This takes place in bone marrow.
- It involves the maturation of lymphoid progenitors to matured naive B cells.
- Antigen dependent phase:
- This takes place in lymph node.
- It involves activation of mature B-cells then they encounter antigen and their differentiation into plasma cells and memory B-cells.
- Antigen independent phase:
B-cell maturation:
- The generation of B-cell first occurs in embryo and continues throughout life.
- Before birth, the yolk sac, foetal liver and foetal bone marrow are the major sites of B cell maturation.
- After birth, the generation of mature B-cells occur in the bone marrow from hematopoietic stem cells (HSC).
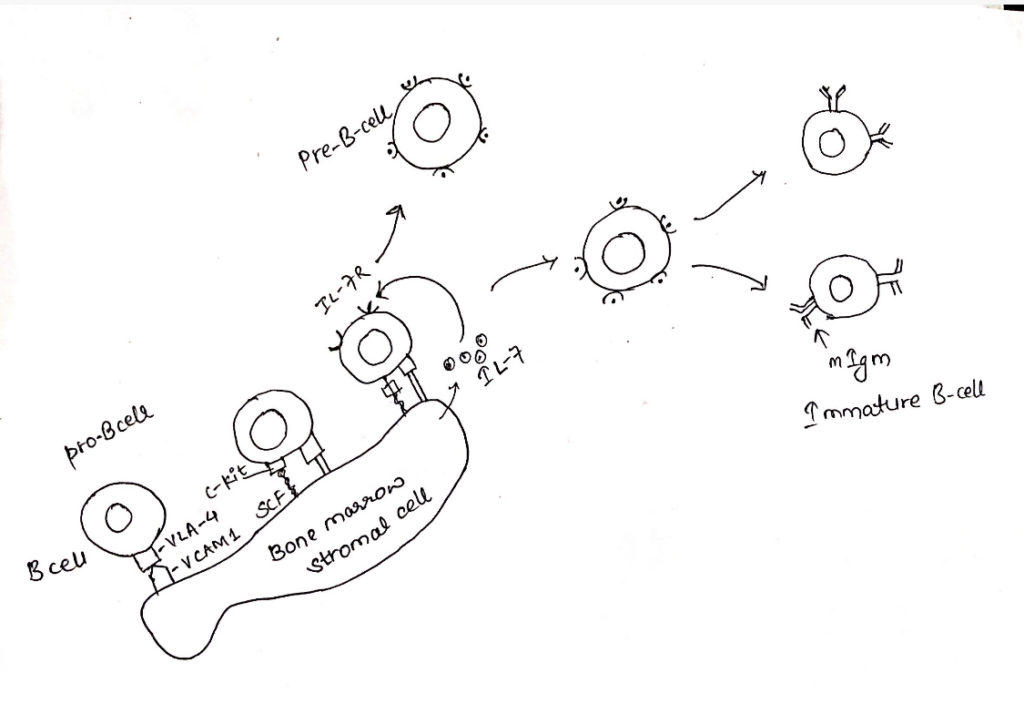
- The HSC first divide to form lymphoid progenitor cells which then differentiate into the progenitor B-cells (pro B) which express a transmembrane tyrosine phosphatase called CD45R and signal transducing molecule Igα/ Igβ which are found associated with the membrane bound antibody in later stages of development.
- Pro-B cell also express CD19 (part of co-receptor), CD43 (leukosialin), CD24 (heat stable), and C-kit are present on the surface of Pro-B-cell.
- The pro-B-cells proliferate within bone marrow filling extravascular spaces between large sinusoids in the shaft of a bone proliferation of pro-B-cells to precursor-B-cells (pre-B-cell) require micro-environment provided by the bone marrow stromal cells.
- The stromal cell plays two important roles, they interact directly with Pro-B cell and Pre-B cell and they secrete various cytokines, notably IL-7 that support developmental process.
- Pro-B-cells need direct contact with stromal cells in the bone marrow during the earliest developmental stage.
- This interaction is mediated by several cell adhesion molecules including VLA-4 on Pro-B cell and its ligand, VCAM-1, on the stromal cell.
- After initial contact is made, a receptor on Pro-B cell called C-kit interacts with a stromal cell surface molecule known as stem cell factor (SCF).
- This interaction activates C-kit, a tyrosine kinase and Pro-B cell begins expressing receptor for IL-7.
- The Pre-B-cell express many of same marker that were present on Pro-B-cell, however they cease to express C-kit and CD43 and begin to express CD25.
- The IL-7 secreted by stromal cells drives the maturation process eventually inducing down the regulation of adhesion molecule on Pre-B cell.
- So, the proliferating cell can detach from stromal cells.
- At this stage, Pre-B-cell no longer requires direct contact with stromal cell but continues to requires IL-7 for growth and maturation.
Ig-gene re-arrangement producing immature B-cells:
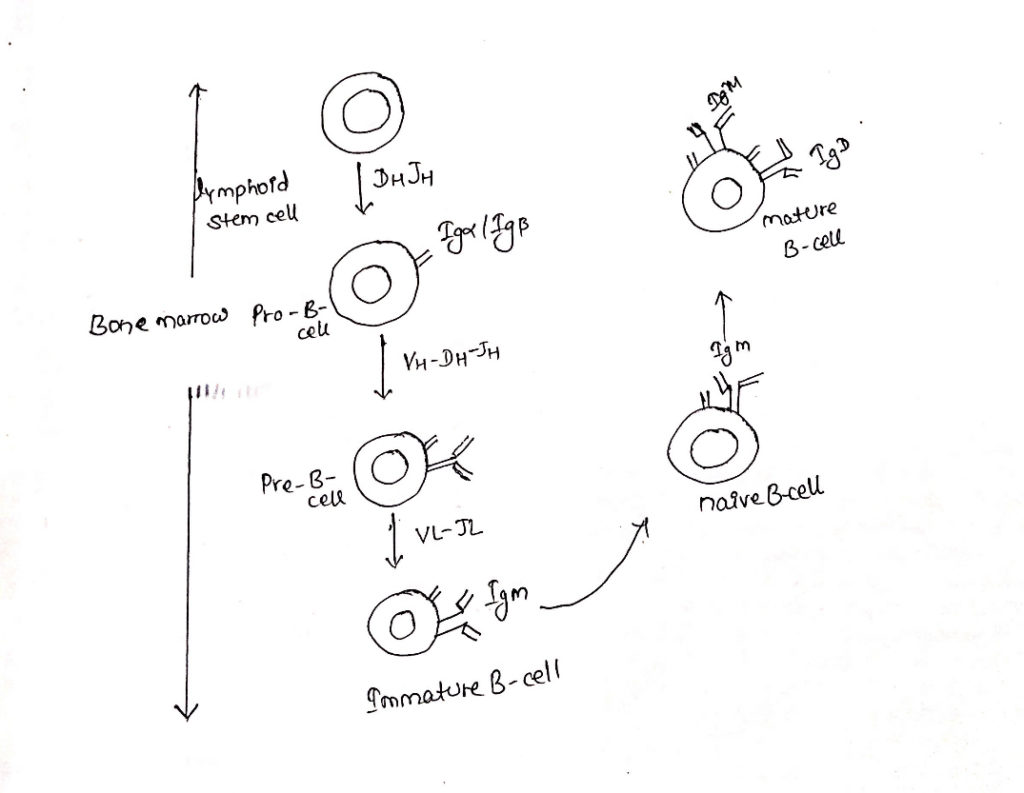
- B-cell maturation depends on rearrangement of immunoglobulin DNA in the lymphoid stem cells.
- The first Ig-gene re-arrangement to occur in Pro-B-cell stage is a heavy chain DH-JH gene re-arrangement, this is VH-DH-JH rearrangement.
- If the first heavy chain rearrangement is not productive, then VH-DH-JH rearrangement continues on the other chromosome.
- Upon completion of heavy chain arrangement, the cell is classified as Pre-B-cell.
- Continued development of a Pre-B-cell into an immature B-cell requires a productive light-chain gene re-arrangement.
- Only one light chain isotype is expressed on the membrane of a B-cell because of allelic exclusion.
- Upon completion of productive light chain re-arrangement, it commits the immature B-cell to a particular antigenic specificity.
- This specificity is determined by the cells heavy chain VDJ sequence and light chain VJ sequence.
- Immature B cell expresses mIgM on its cell surface.
- The bone marrow phase of B-cell development culminates in the production of IgM bearing immature B-cell.
- At this stage of development, B-cell is still not fully functional.
- Thus, antigen induces death or unresponsiveness rather than division and differentiation.
- The co-expression of IgD and IgM on the membrane signals the full maturation.
- This progression involves a change in RNA processing of the heavy chain primary transcript to permit the production of two mRNAs, one encoding the membrane form of the µ chain and other encoding the membrane of the 𝛿 chain.
B-cell proliferation and activation:
- After export of B-cell from the bone-marrow, activation, proliferation and differentiation occur in the periphery and require antigen.
- Depending on the nature of the antigen, B cell activation proceeds by two different routes, one dependent of TH cell, the other not.
- The B cell response to thymus dependent (TD) antigen requires direct contact with TH cell, not simply exposure to TH derived cytokines.
- Antigens that can activate B cells in absence of this kind of direct participation by TH cells are known as thymus independent (TH) antigen.
- The TI antigens are divided into two types 1 and 2 and they activate B-cells by different mechanisms.
- Most TI1 antigens are polyclonal B cell activator i.e. they are able to activate B-cell regardless of their antigenic specificity.
- At high concentration TI-1 antigens will stimulate proliferation and antibody secretion by as many as one third of B-cells.
- It includes bacterial cell wall components including lipopolysaccharide.
- B cells are activated by TI-2 antigens by extensively crosslinking the mIg receptor.
- However, TI-2 antigens contrasts to TI-1 antigens in three important respects.
- First, they are not B-cell mitogens and do not act as a polyclonal activators.
- Second, TI-1 antigens activate both mature B-cells and immature B cells. Whereas TI-2 antigen activates mature B cells and inactivates immature B-cells.
- Third, although B cell response to TI-2 antigen does not require direct involvement of TH cells, cytokines derived from TH cells are required for efficient B-cell proliferation and for class switching to isotypes other than IgM.
- It includes highly repetitious molecules like bacterial flagellin.
- Activation of B-cell by soluble protein antigen requires the involvement of TH cells.
- Binding of antigen to B-cell mIg does not itself induce on effective competence without additional interaction with membrane molecule on the TH cell.
- In addition to it, a cytokine mediated progression is required for B-cell proliferation.
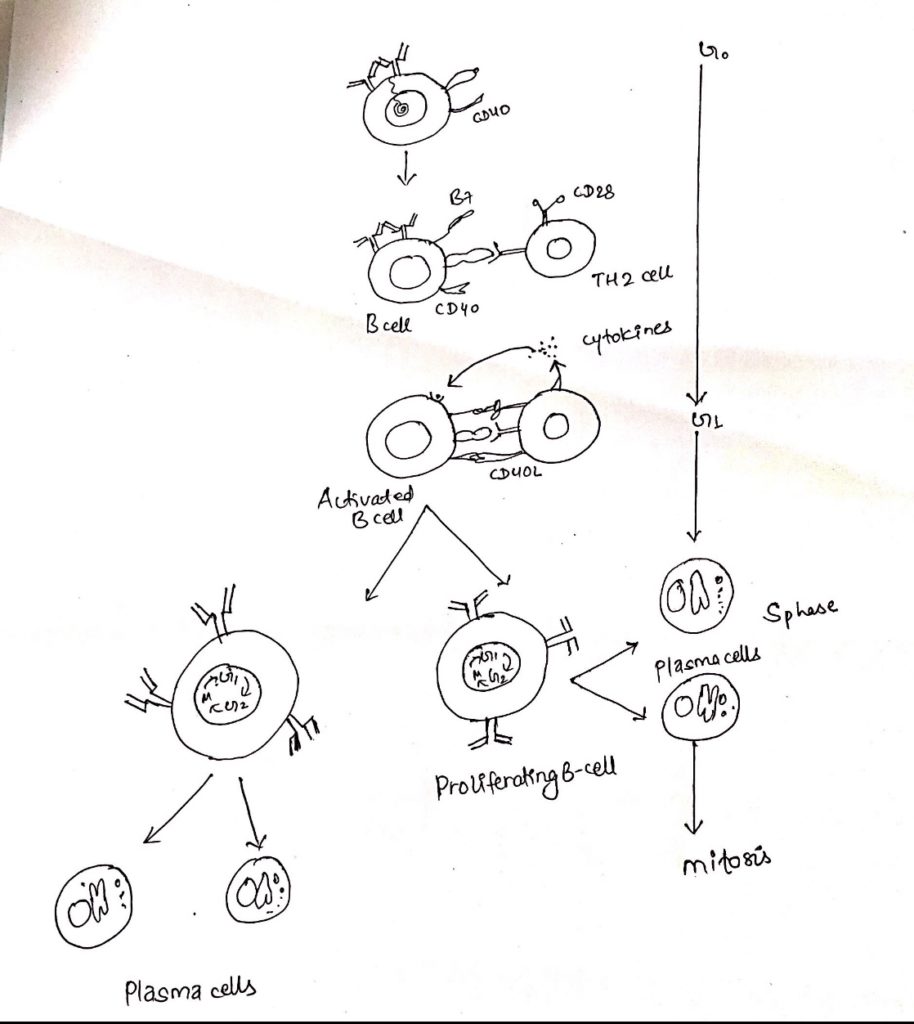
i. Formation of T-B conjugate:
- After binding of antigen by mIg on B cell, the antigen is internalized by receptors mediated endocytosis and processed within the endocyte pathway into peptide.
- Antigen binding also initiates signaling through the BCR that induces the B-cells to upregulate a no. of cell membrane molecules, including class II MHC molecules and co-stimulatory ligand B7.
- Increased expression of both of these membrane proteins enhance the ability of B-cell to function as an antigen presenting cell (APC) in TH cell activation.
- Once the TH cell recognizes a processed antigenic peptide displayed by a class II MHC molecule on the membrane of B-cells, the two cells interact to form a T-B conjugate.
- This structural adjustment facilitates the release of cytokines towards the antigen specific B-cells.
ii. CD40-CD40L interaction:
- Formation of a T-B conjugate not only leads to directional release of TH cell cytokines but also to the upregulation of CD40L, a TH cell membrane protein that then interacts with CD40 on the B-cell to provide essential signal for T-cell dependent B-cell activation.
- Interaction of CD40L with CD40 on the B-cell delivers a signal (signal-2) to the B-cell that in cooperation with the signal generated by mIg cross-linkage (signal1), drives the B cell into G1.
- The signal from CD40 are transducted by a no. of intracellular signaling pathway, ultimately resulting in changes in gene expression.
iii. Signals provided by TH cell cytokines:
- The antigenic specific interaction between a TH and a B cell induces a redistribution of TH cell membrane proteins and cytoskeletal elements that results in the polarized release of cytokines towards the interacting B-cell.
- Once the B cell becomes activated, it begins to express membrane receptors for various cytokines such as IL-2, IL-4, IL-5 and others.
- These receptors then bind the cytokines produced by these cytokine-receptor interaction support B-cell proliferation and can induce differentiation, proliferation and can induce differentiation into plasma cells and memory cells, class switching and affinity maturation.
- Antigen crosslinking mIg, generating signal 1 which results in increased expression of class II MHC and co-stimulatory B7. Antigen-antibody complexes are internalized by receptor mediated endocytosis. Then it is degraded to peptides, some of which are bound by class II MHC and presented on the membrane as peptide-MHC complexes.
- TH cell recognizes antigen-MHC-II on B-cell membrane. This plus co-stimulatory signal activates TH cell.
- i. TH cell begins to express CD40L
ii. Interactions of CD40 and CD40L provides signal 2 - iii. B7-CD28 interactions provide co-stimulation to the TH cell.
- i) B-cell begins to express receptors for various cytokines.
ii) Binding to cytokines released from TH cell in a directed manner relays signal that the progression of the B-cell to DNA synthesis and to differentiation.