
Benedict’s Test: Objective, Principle, Reagents, Procedure and Result
Objective:
- to detect reducing sugar ( carbohydrate having free aldehyde or ketone functional group)
Principle:
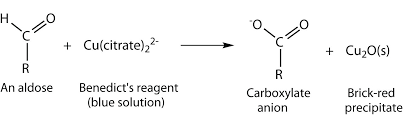
The Reducing sugar under alkaline condition form enediols. Benedict’s solution contains milder alkali Na2CO3. Enediols are powerful reducing agents. They can reduce cupric ions to cuprous ions which is the basis for Benedict’s reaction. The cuprous hydroxide during the process of heating is converted to red cuprous oxide.
Reagents:
- test solutions: 5 % Glucose, 5 % Sucrose
- Benedict’s reagent: CuSO4.5H2O solution with Sodium carbonate and sodium citrate
- Water bath
- Dry test tubes
- Pipettes
Procedures
- Take 1ml of test sample in dry test tube.
- Take 1ml of distilled water in another tube as control.
- Add 2ml of Benedict’s reagent to all the tubes.
- Keep in water bath for 5 minutes.
- Look for the development of brick red precipitate.
Result interpretation:

- positive benedict’s test: color change from blue to brick red ppt ( glucose)
- Negative Benedict’s test: no change in color( sucrose)
| Color observed | Sugar % | Result interpretation |
| Blue | Nil | Absent of suagr |
| Green color | 0.5% | + |
| Green ppt | 0.5-1% | ++ |
| Yellow ppt | 1-1.5% | +++ |
| Orange ppt | 1.5-2% | ++++ |
| Brick red ppt | >2% | +++++ |
