
Strongyloides stercoralis
- Strongyloides stercoralis is commonly known as dwarf threadworm.
- It was identified by Baray in 1876.
- It is the smallest nematode known to cause infection in humans.
- The parasite is unique in that it has both parasite and free living form
Habitat:
- The parasite females live in the mucus membrane of small intestine of human especially in the duodenum and jejunum.
- The female worm lie in the tunnels between the enterocytes of the intestinal mucosa.
Morphology
- Strongyloides stercolaris exist in both parasitic and free living form.
- In the parasite phase, the females are readily discovered but not the males.
1. Parasitic female
- Female measure 2500 µm in length and 40-50 µm in breadth and are transparent.
- The buccal cavity has four small lips.
- The cylindrical muscular oesophagus extends through the anterior third of the body and the intestine extends through the posterior two thirds.
- The anus appears mid ventrally, a short distance in front of the caudal tip. The posterior end is extremely pointed.
- A paired uterus, oriduct and ovaries constitute the female genital organs. The vulval opening is present at the junction of the middle and posterior part of the body.
- The females are Ovo-viviparous. Each female lays upto 30-40 partially embroynated eggs per day in the mucosal epithelium of the intestine of human.
2. Parasitic males
- They are shorter and broader than females.
- They have no penetrating power and remain parasitic in the lumen of large intestine.
- Parasitic male donot exist in infected humans.
- Males differ from females by possessing spicules and gubernaculum.
3. Eggs
- In the gravid females, the eggs are conspicuous within its body lying antero-posteriorly in a single file (5-10 eggs).
- Eggs measure 55 µm in length and 30 µm in breadth.
- They are thin-shelled, transparent and oval. They contains larva ready to hatch.
- As soon as the eggs are laid, the rhabditiform larva start hatching and bore their way to the lumen of the intestine from where they are excreted out with faeces.
4. Larva:
- The larva of S. Stercoratis is of two types:
i. Rhabditiform larva (first stage larva):
- It is the first stage larva which immediately hatch out of the eggs laid by the gravid female in the mucosa of the small intestine.
- They are actively motile, measuring 200-300 µm in length and 16 µm in breadth.
- They have a short mouth and double bulb oesophages and an inconspicuous genital primordium.
ii. Filariform larva:
- They are longer and more slender measuring 630 µm in length and 10 µm in breadth.
- They have short mouth and long cylindrical oesophagus.
- This larva is the infective stage of the parasites.
Life cycle of Strongyloides stercoralis:
- Life cycle is completed in as single host, principally humans.
- The life cycle is unique due to its potential for auto infection and multiplication within the infected hosts.
- The parasite shows two distinct life cycles, one within the human body and other free living in the soil.
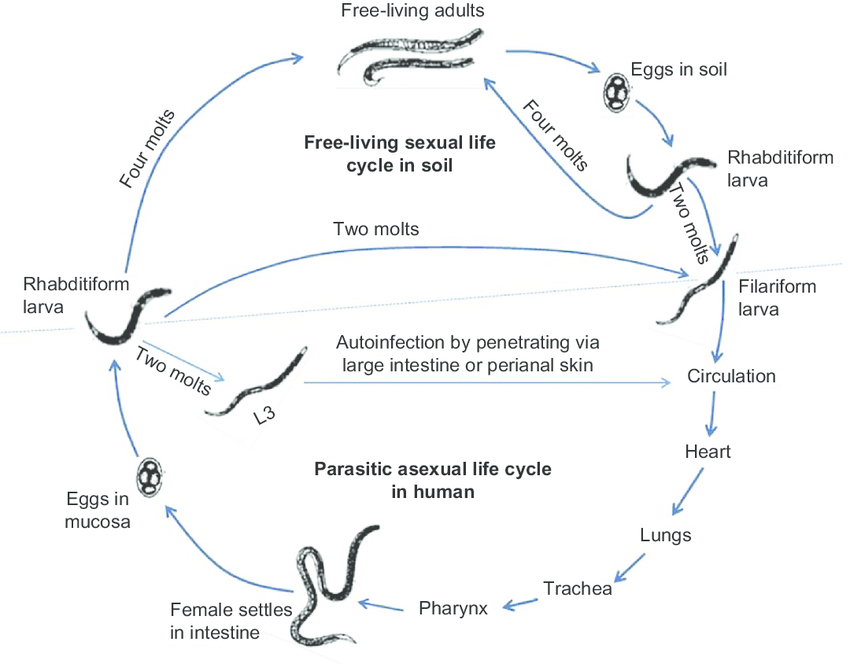
Cycle in human host:
- Man acquire infection mainly through penetration of the skin and occasionally through buccal mucosa by the infective filariform larva.
- The larva invade the tissue, penetrate the venous circulation and are carried by the blood stream to the right heart and then to the lungs. They leave the pulmonary capillaries and enter the lung then they migrate to the bronchi, trachea, larynx and epiglottis and are swallowed back and enter the intestinal tract. Here the larva mould twice and become adult female worm.
- The female burrow deep into the intestinal mucosa and lay eggs by parthenogenesis. The eggs hatch to produce non infective larva. This larva may then progress to parasitic cycle or the free living cycle.
Parasitic cycle or Autoinfection cycle:
- While still in the intestine the rhabditiform larva may be metamorphosed into filariform larva.
- These larva penetrate the intestinal mucosa causing internal autoinfection or the skin of perianal area causing external infection.
- In this ways the infection is continued by separated migratory cycle throughout the life of infected host.
- If the larva are carried to the bowel they may be voided with the faeces.
- Auto infection is an unique feature of the parasite.
Free living cycle:
- In the soil, the rhabditiform larva deposited with the faeces can either develop into infective filariform larva or to new free living adults.
Direct cycle:
- The rhabditiform larva in the soil develop into the second-stage rhabditiform larva and finally undergo a second mould to form the infective filariform larva within 3-4 days.
- This larva then penetrate the skin of lumen and the cycle is repeated.
Indirect cycle:
- The rhabditiform larva matures in course of 24-30 hours to free living adult male and female.
- Copulation between them, resulting in production of second batch of rhabditiform larvae. Each pair of rhabditiform larva from first batch gives rise to nearly 30 filariform larva.
- In 3-4 days, these larvae are transformed to filaniform larva.
Mode of transmission:
- Soil contaminated with human faeces is the main source of infection.
- Human acquires infection-
- Commonly, by penetration of the skin by the filariform larva
- Ingestion of food and drink contaminated with larva.
- By organ transplant such as kidney, transplanted to a new host.
- Less commonly, by transmission of the larva from mother to the infant through the milk.
Pathogenesis of Strongyloides stercoralis:
- Both the larva and adults are pathogenic
Pathogenicity of larva:
- Infective filariform larva at the site of invasion produce macules and papules.
- In the host sensitized earlier to strongyloides antigens, the larva causes allergic reaction such as urticaria and pruritus.
- In the lungs, larva produces a considerable degree of tissue damage and injury to the alveoli and bronchial epithelium, thereby producing bronchopneumonia or full blown pneumonitis.
- It produces inflammatory exudates of macrophages, epithelial cells and minimal hemorrhage.
Pathogenicity of adult worm:
- In the intestinal mucosa of the duodenum and jejunum the adult worms produce mild to moderate degree of oedema, cellular infiltration and partial villous atrophy.
- In severe infection ulcers and long standing infectious fibrosis is found occasionally.
- In autoinfection and hyperinfection syndrome, the mucosal inflammation is severe. The sigmoidal colon and rectum frequently becomes thickened and oedematous. The cellular reactions around the larvae are typically absent.
Clinical symptoms of Strongyloides infection:
- S. Sterecoralis causes strongyloidiasis.
- Most infection in human are asymptomatic.
- In case of symptomatic cases, following infection are seen-
i. Cutaneous infection:
- This phase corresponds to invasion of the skin by filariform larva.
- Two types of lesions are seen
- An intestinal lash at the site of infection
- A linear erythematous urticarial wheal around the anus caused by migrating filiform larva. This condition is called larva currens.
ii. Pulmonary infection:
- Hemorrhages in the lungs alveoli and bronchopneumonia is observed during migration of filariform larva through lungs and form an avenue of escape into the alveoli.
- Alveoli infiltrated with eosinophil cells.
- Dyspnea, wheezing, low grade fever and at times productive cough with blood streaked sputum are the frequent symptoms
iii. Intestinal infection:
- The intestinal lessons are produced due to the invasion of the intestine by the adult worms.
- Profuse watery and mucoid diarrhea is the classical presentation of acute stronglyloidiasis.
- Epigastric abdominal discomfort, indigestion and occasionally, nausea and vomiting are other symptoms.
- In chronic cases diffuse abdominal pain, nausea, vomiting, hyperactive or hypo reactive absent bowel sounds and diarrhea occasionally bloody are frequently seen.
- Immunosuppressive patients with strong strongyloides infection may develop massive strongyloidiasis called hyperinfective syndrome. It manifest as severe diarrhea, malabsorption, paralytic peritonitis, meningitis, brain absess.
- Filariform larva may act as vehicle of microbial infection leading to Gram-negative bacteriaemia.
iv. Other systematic infection:
- Cardiac arrhythmias
- Myocardial damage caused by the migrating larva
Epidemiology and geographical distribution
- S. stercoralis is worldwide in distribution.
- It is endemic in the tropics and sub tropics than the temperate countries.
- The parasite is most prevalent in West Africa, South America, Brazil and Southeast Asia.
Laboratory diagnosis of Strongyloides:
1.Specimens:
- Stool, urine and sputum
- A specific diagnosis is based upon the finding of the typical rhabditiform larva in freshly passed stool.
2. Methods of examination
i. Stool microscopy:
- Only the larva is demonstrated, never the eggs.
- The larva resemble that of hookworms. They are distinguished by their shorter buccal cavity, notched-tail and oesophagus present in one half of the body.
- The larva in the stool can be concentrated via the formalin-ether or Zinc floatation methods.
ii. Stool culture:
- If the larva are scanty in faeces, culture is useful.
- Stool can be cultured either by Mori filter paper method or Baemann funnel method using charcoal or the agar plate method.
- The agar plate method is most sensitive method.
iii. Enterotest:
- The rhabditiform larva in the intestinal fluid aspirated can be demonstrated duodenal intubations by Enterotest or sting test.
- In case of disseminated strongyloidiasis the larva can also be found in sputum, bronchial washings, bronchoalveolar lavage and sometimes in CSF.
iv. Sero-diagnosis:
- ELISA, IHA and IFA are useful for the diagnosis of the disease. However, these serological test cannot distinguish between recent and old infections.
v. Skin test
vi. Imaging methods: x-rays, Ct scan
vii. Hematology test:
- Eosinophilia (> 500/mm3 of blood) is demonstrated in acute and chronic strongyloidiasis.
Treatment for Strongyloides infection:
i. IVERMECTIN
- It is a drug of choice.
- Dose- 2 mg/kg weight in four divided for a period of 2 days
- It is an oral drug
ii. ALBENDAZOLE, THIALBENDAZOLE, MEBENDAZOLE.
Prevention and control measures
- Sanitary disposal of faeces
- Improved personal hygiene
- Treatment of infected persons.
