Widal test: Introduction, Principle, Procedure, Result interpretation, Applications and limitations
Introduction
- Widal test is a serological test which is used for the diagnosis of enteric fever or typhoid fever. The test was developed by Greembaum and Widal in 1896. Typhoid or enteric fever is caused by a gram negative bacteria Salmonella enterica (Salmonella Typhi or Salmonella Paratyphi), found in the intestine of man. Salmonella paratyphi also causes Typhoid but of a milder form.
- Salmonella possess O antigen on their cell wall and h antigen on their flagella. On infection, these antigen stimulates the body to produce specific antibodies which are released in the blood. The Widal test is used to detect these specific antibodies in the serum sample of patients suffering from typhoid using antigen-antibody interactions. These specific antibodies can be detected in the patient’s serum after 6 days of infection (fever).
- Salmonella Typhi possesses O antigen on the cell wall and H antigen on flagella. Salmonella Paratyphi A and S. Paratyphi B also possess O antigen on their cell wall and but have AH and BH antigen on their flagella respectively.
Principle of Widal test:
- Widal test is an agglutination test in which specific typhoid fever antibodies are detected by mixing the patient’s serum with killed bacterial suspension of Salmonella carrying specific O, H, AH and BH antigens and observed for clumping ie. Antigen-antibody reaction. The main principle of Widal test is that if homologous antibody is present in patient’s serum, it will react with respective antigen in the suspension and gives visible clumping on the test slide or card.
Requirements for widal test:
i) Fresh serum, stored at 2-8° Serum should not be heated or inactivated.
ii) The complete kit containing five vials containing stained Salmonella antigen
- S. Typhi———-O antigen
- S. Tyhhi———- H antigen
- S. Paratyphi —–AH antigen
- S. Paratyphi —–BH antigen
iii) Widal positive control
iv) Widal test card or slide
v) Applicator stick
Procedure of Widal test:
- Widal test can be done in two ways-one is rapid test on slide and another is tube test in which result may be obtained after one night of incubation.
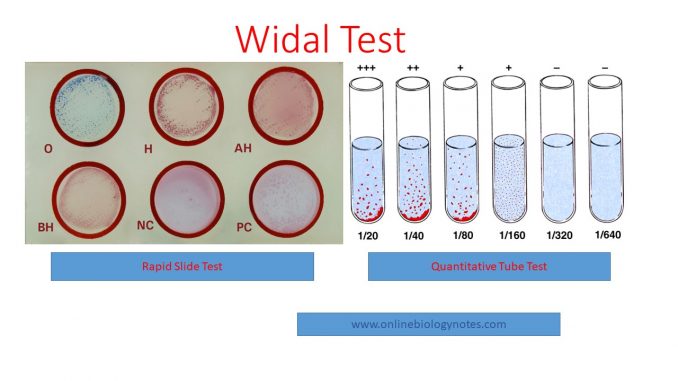
I. Rapid slide test:
- Clean the glass slide or test card supplied in the kit well and make it dry.
- Label the circles (1, 2, 3, 4, 5 and 6) in the test card as O, H, AH, BH, Negative control and Positive control
- Place a drop of undiluted test serum in each of the four labelled circle (1, 2, 3 and 4) ie O, H, AH and BH and place a drop of Negative control serum in circle 5 and Positive control in circle 6.
- Place a drop of antigen O, H, AH and BH in circle 1, 2, 3, and 4 respectively and no antigen in circle 5 and O/H antigen in circle 6.
- Mix the content of each circle with a separate wooden applicator stick and spread to fill the whole area of the individual circle.
- Rock the test card for a minute and observe for agglutination.
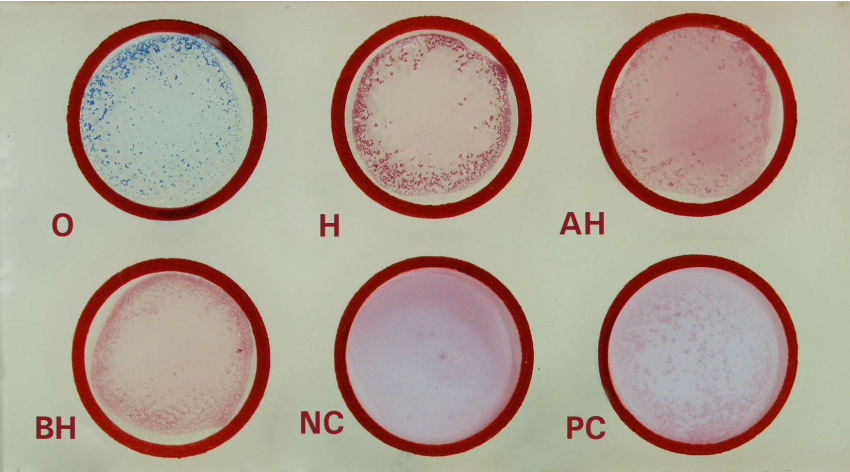
*if agglutination is visible within 1 minute, proceed for quantitative slide test or tube test for the quantitative estimation of the titre of the antibody.
II. Quantitative slide test:
- Clean the test card and make it dry
- Put 0.005 ml, 0.001 ml, 0.02ml, 0.04ml and 0.08ml of undiluted serum in 1st, 2nd, 3rd, 4th and 5th circles respectively in the test card.
- Add a drop of appropriate antigen suspension which showed agglutination in rapid slide test, to each of the above circles.
- Mix the contents of each circle with a separate wooden applicator stick.
- Rock the slide slowly for 1 minute and observe for agglutination.
- The titre of the antibody is the highest dilution of serum up to which there is clear agglutination.
- Repeats steps 1 to 6 with all the antigens, which showed agglutination in rapid slide test.
The serum volumes in the quantitative slide test corresponds approximately to the tube test is given below:
| Circle | Serum volume | Antigen drop | Approx. test tube titre |
| 1st | 0.08 ml | 1 drop | 1 : 20 |
| 2nd | 0.04 ml | 1 drop | 1 : 40 |
| 3rd | 0.02 ml | 1 drop | 1 : 80 |
| 4th | 0.01 ml | 1 drop | 1 : 160 |
| 5th | 0.005 ml | 1drop | 1 : 320 |
III. Quantitative tube test:
- Take a set of 8 clean dry test tubes (Kahn tubes) and label as 1, 2,3, 4, 5, 6, 7 and 8 for O antibody detection.
- Similarly, take 3 sets of 8 test tubes and label then as 1, 2…8.
- Dilute the serum samples as follows:
- Pipette into the tube No.1 of all sets 1.9 ml of isotonic saline.
- To each of the remaining tubes (2 to 8) add 1.0 ml of isotonic saline.
- To the tube No.1 tube in each row add 0.1 ml of the serum sample to be tested and mix well.
- Transfer 1.0 ml of the diluted serum from tube no.1 to tube no.2 and mix well.
- Transfer 1.0 ml of the diluted sample from tube no.2 to tube no.3 and mix well. Continue this serial dilution till tube no.7 in each set.
- Discard 1.0 ml of the diluted serum from tube No.7 of each set.
- Tube No.8 in all the sets, serves as a saline control. Now the dilution of the serum sample achieved in each set is as follows: Tube No. : 1 2 3 4 5 6 7 8 (control) Dilutions 1:20 1:40 1:80 1:160 1:320 1:640 1:1280.
4. Add a drop of appropriate widal test antigen to all the test tubes
5. Mix well and incubate at 37°C for 16-20 hours and examine for agglutination.
6. Antibody titre is the highest dilution of serum showing clear agglutination.
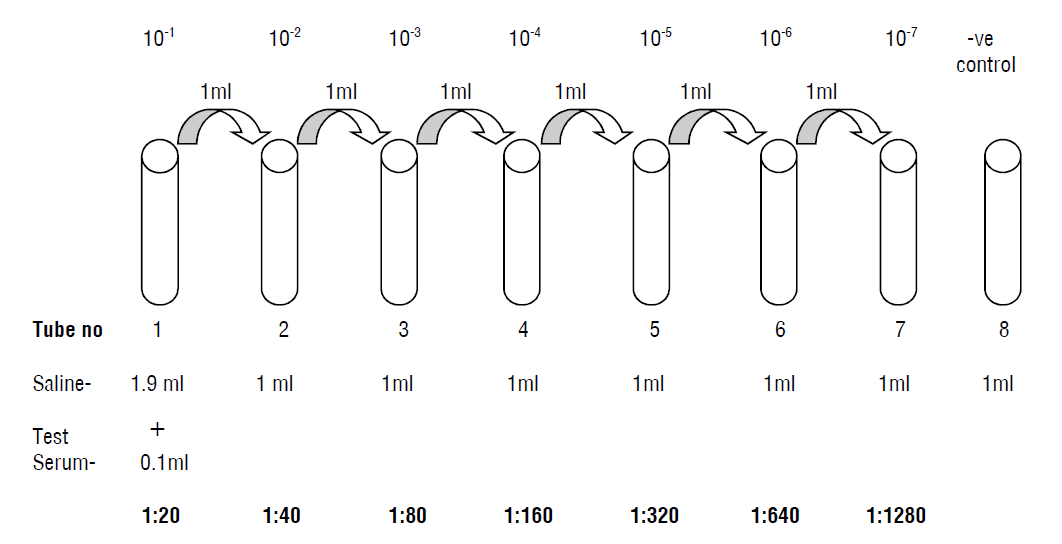
| Test tube | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Dilution | 1 : 20 | 1 : 40 | 1 : 80 | 1 : 160 | 1 : 320 | 1 : 640 | 1 : 1280 | Control (saline) |
Result interpretation of Widal test:
- Antibody titre greater than 1 : 80 is considered significant and usually suggests positive test for Salmonella infection.
- Low titres are often in normal individuals
- A single positive is less significant than the rising antibody titre, since rising titre is considered to be a definite evidence of infection.
Applications of Widal test:
- Rapid test for screening typhoid fever in endemic areas.
- When culture facilities is not available, Widal test is very reliable
- Use for both Salmonella Typhi and Salmonella Paratyphi
Limitations of Widal test:
- Widal test is time consuming to find antibody titre and often times when diagnosis is reached it is too late to start an antibiotic regimen.
- Widal test may be falsely positive in patients who have had previous vaccination or infection with S.Typhi.
- Widal test cannot distinguish between a current infection and a previous infection or vaccination against typhoid.
- Widal test shows cross-reactivity with other Salmonella species.
- False positive Widal test results are also known to occur in typhus, acute falciparum malaria
(particularly in children), chronic liver disease associated with raised globulin levels and disorders such as rheumatoid arthritis, myelomatosis and nephrotic syndrome. - Widal test should be interpreted in the light of baseline titers in a healthy local population. The antibody levels found in a healthy population however, may vary from time to time and in different areas, making it difficult to establish a cut off level of baseline antibody in a defined area and community.
- Severe hypoproteinaemia may also prevent a rise in 0 and H antibody titres. False negative Widal tests may be due to antibody responses being blocked by early antimicrobial treatment or following a typhoid relapse.
- In low typhoid endemic areas, weak and delayed O and H antibody responses limit the usefulness of the Widal test. Variations also exist between laboratories in the performance and reading of Widal tests which compromise further the reliability of the test.
- The World Health Organization (WHO) has said that due to the various factors that can influence the results of a Widal test, it is best not to rely too much on this test.
